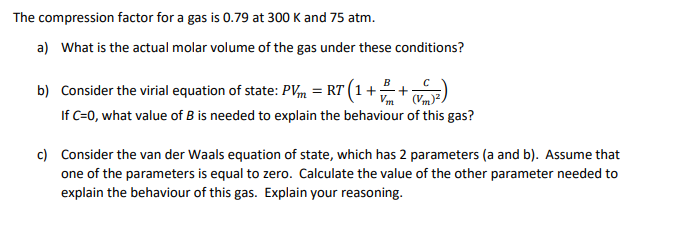
UNUB At Boyle temperature, the value of compressi factor Z has a

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

Determine Compressibility of Gases

What is compressibility factor? What is its value for ideal gas

qph.cf2.quoracdn.net/main-thumb-56835184-200-zlplu

At Boyle's temperature , compressibility factor Z for a real gas is
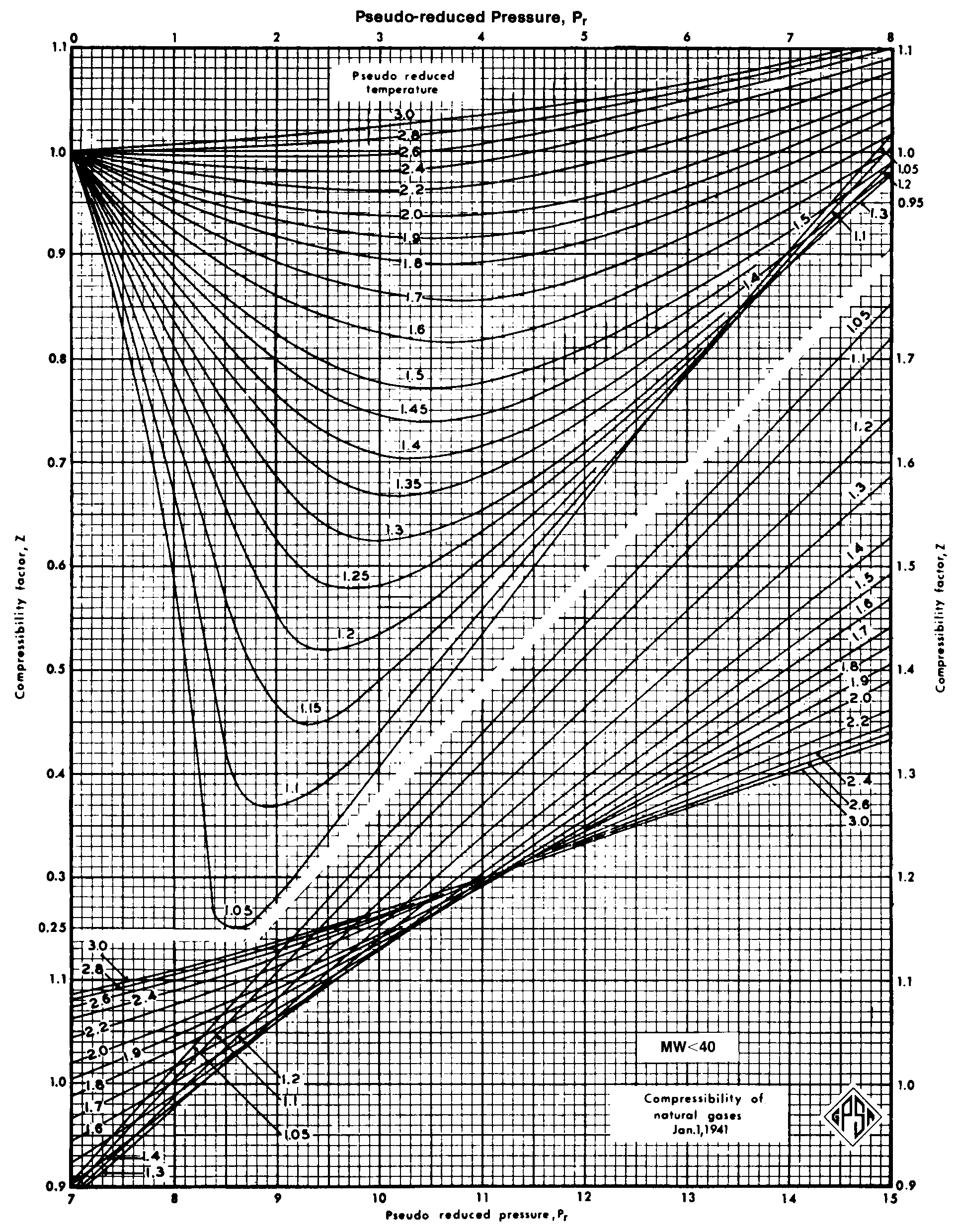
Determine Compressibility Factor, Z Factor - Engineering Units
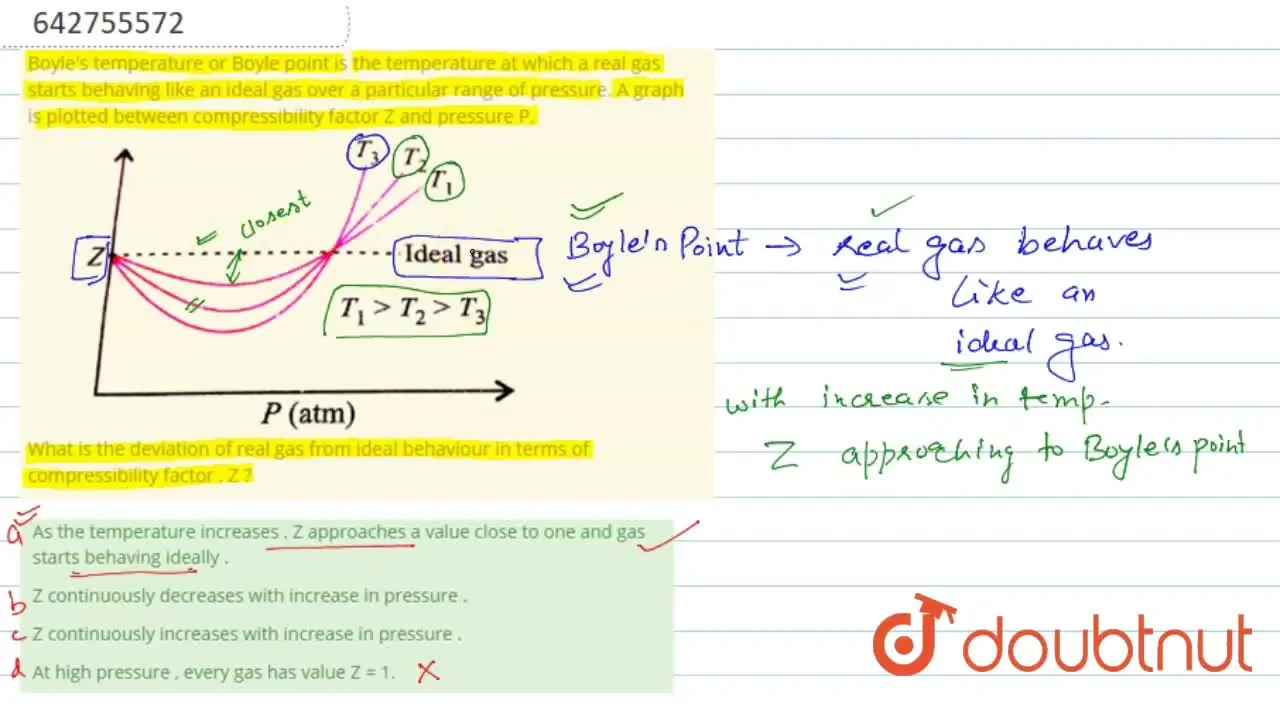
Z continuously increases with increase in pressure .

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Solved 1) The compression factor, Z, can be written as: Z =
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is

1.7: Connecting the van der Waals and the viral equations- the Boyle temperature - Chemistry LibreTexts

68. The compressibility factor (z) an ideal gas is equal to which of the following values? (A) Zero (B) Less than one (C) Equal to one

qph.cf2.quoracdn.net/main-thumb-294940393-200-cueh

Deviation From Ideal Gas Behavior - Study Material for IIT JEE