The compression factor (compressibility factor) for 1 mol of a van der

By A Mystery Man Writer
For 1 mol of a gas, the van der Waals equation is (P+(a)/(V(m)^(2)))(V(m)-b)=RT Ignoring b, we get (given volume of gas molecule is negligible) (P+(a)/(V(m)^(2)))V(m)=RT ltbgt or pV(m)+(a)/(V(m))=RT or (pV(m))/(RT)+(a)/(V(m)RT)=1 or Z=(pV(m))/(RT)=1-(a)/(V(m)RT) (i) It is given that Z=(pV(m))/(RT)=0.5implies V(m)=(0.5RT)/(P) With this, equation (i) becomes 0.5=1-(a)/((0.5RT//p)RT) or a=(0.5)((0.5RT)/(p))RT=0.25(R^(2)T^(2))/(p) Substiuting the given values, we get a=(0.25)[((0.082L atm K^(-1)mol^(-1))^(2)(273 K)^(2))/((100 atm))] =1.2528 L^(2) atm mol^(-2)
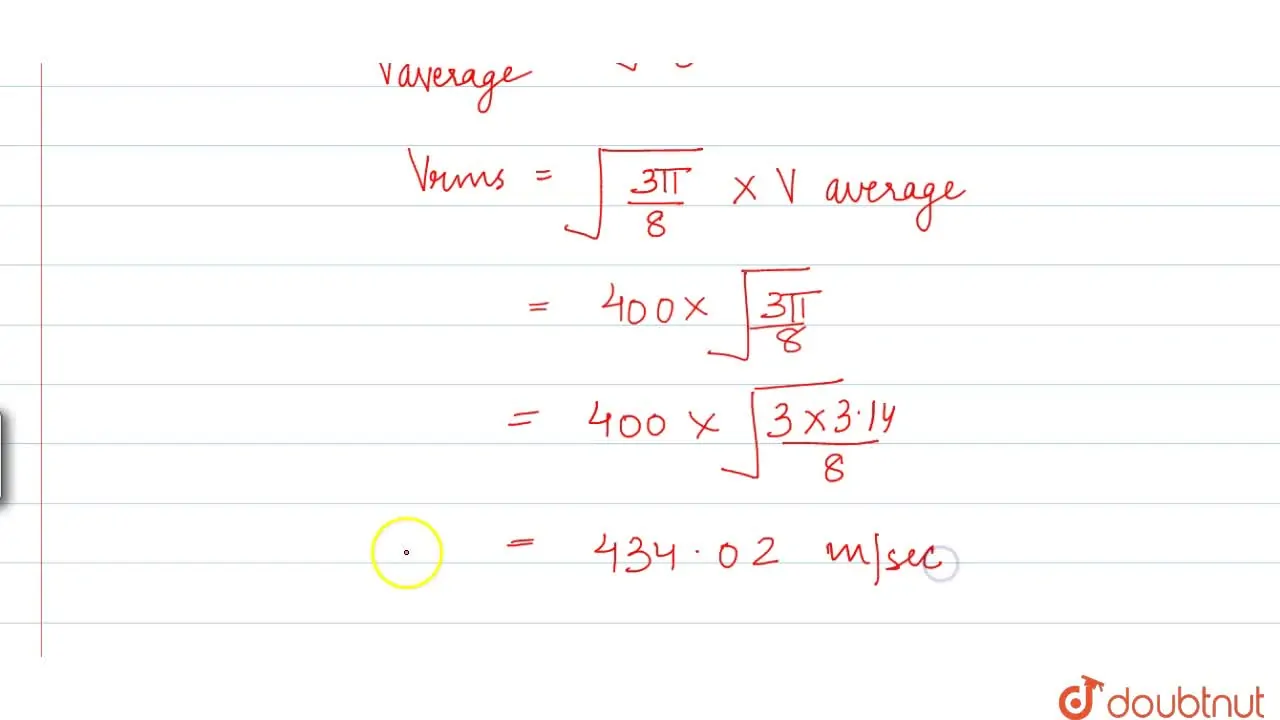
The average velocity of gas molecules is 400 m s^(-1). Calculate their
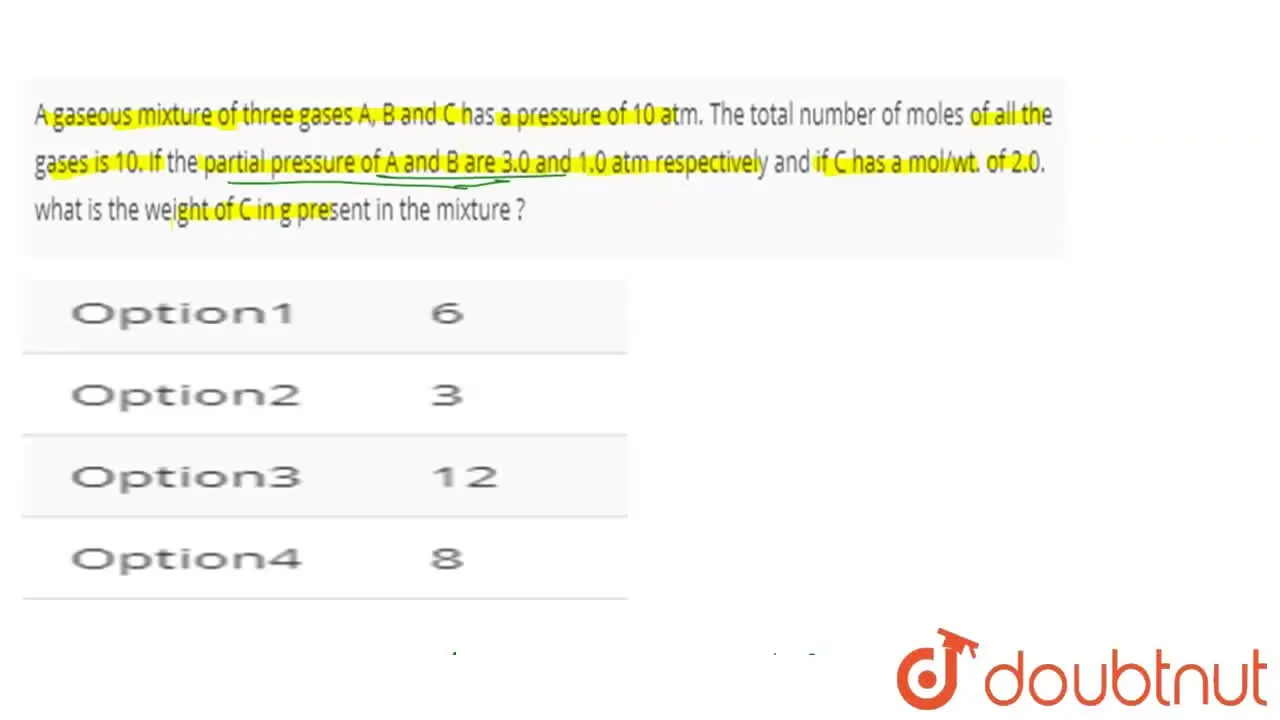
A gaseous mixture of three gases A, B and C has a pressure of 10 atm.

The compressibility factor for definite amount of van der Waals' gas a

Longest mean free path stands for
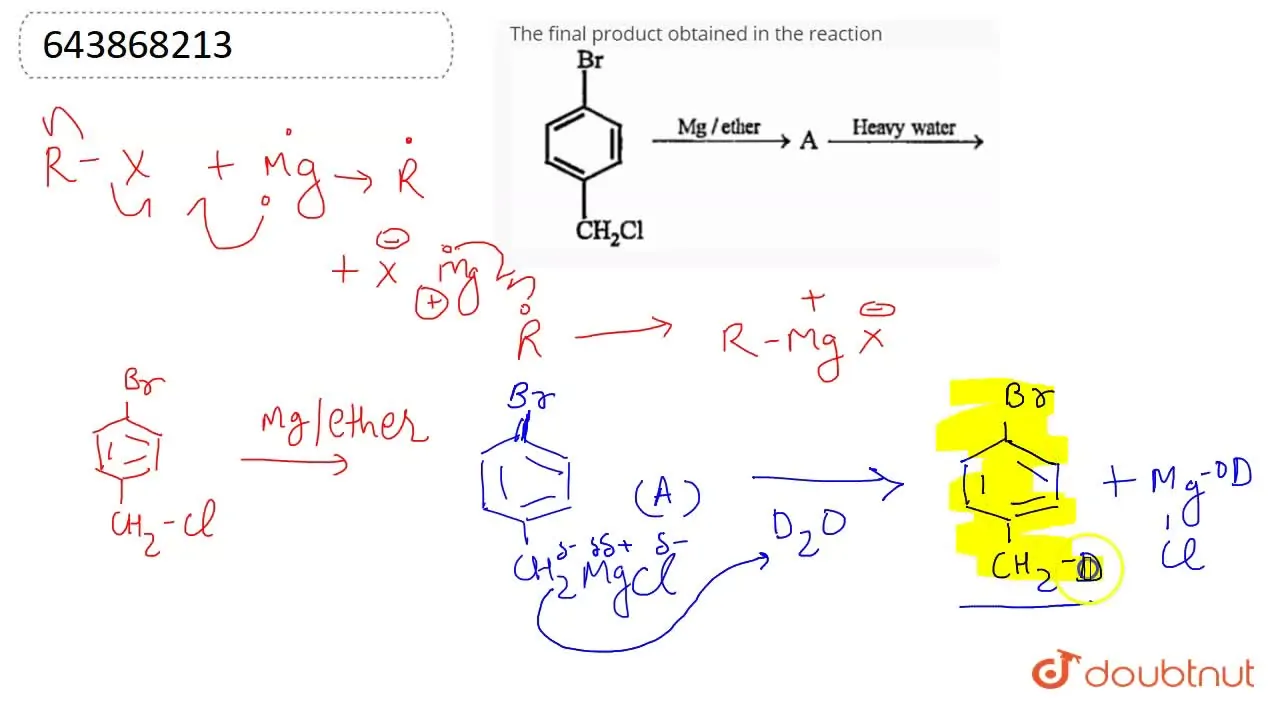
The final product obtained in the reaction

Two reactants A and B separately shows two chemical reactions.Both rea
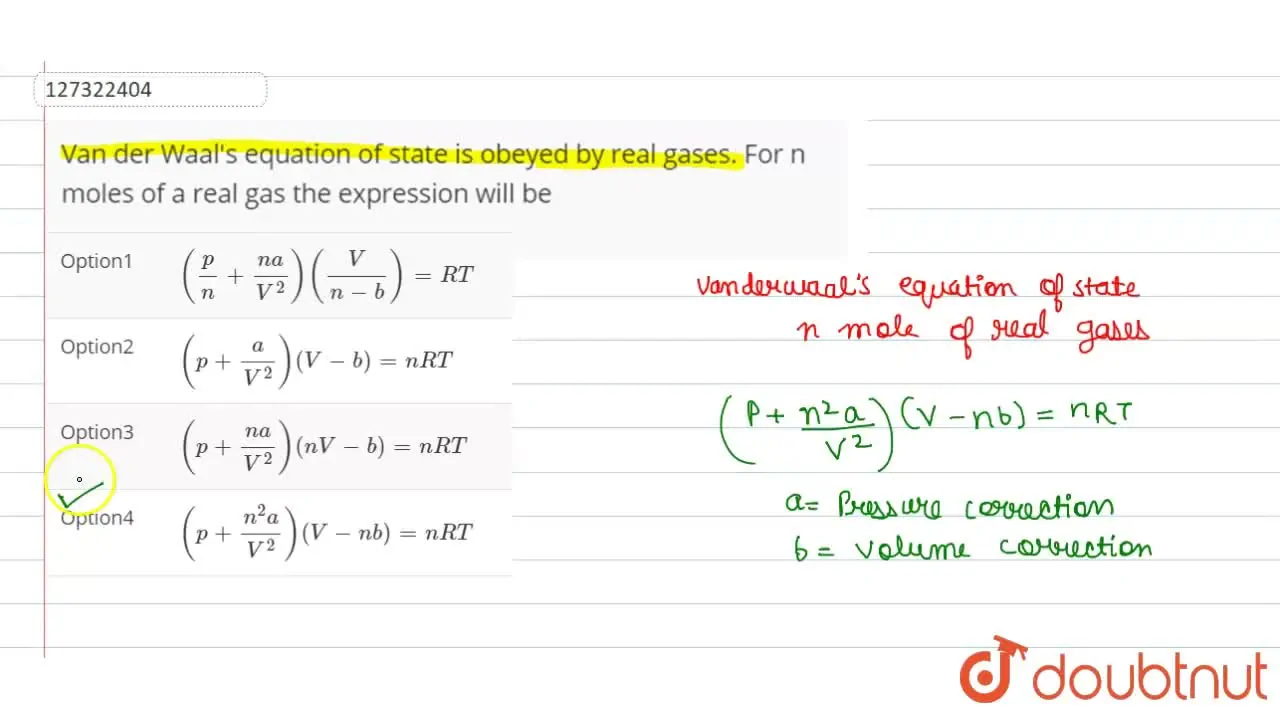
Van der Waal's equation of state is obeyed by real gases. For n moles
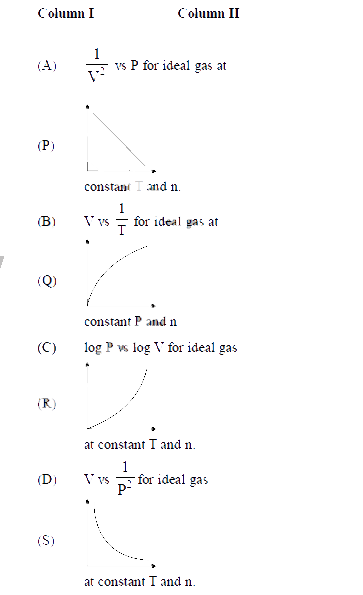
Match the entries in column I with entries in Column II and then pick

Bengali] Compressibility factor of 2 mol of NH(3) gas at 27^(@)C and
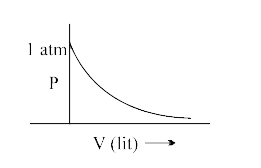
On the recently discovered 10^(th) planet it has been found that the
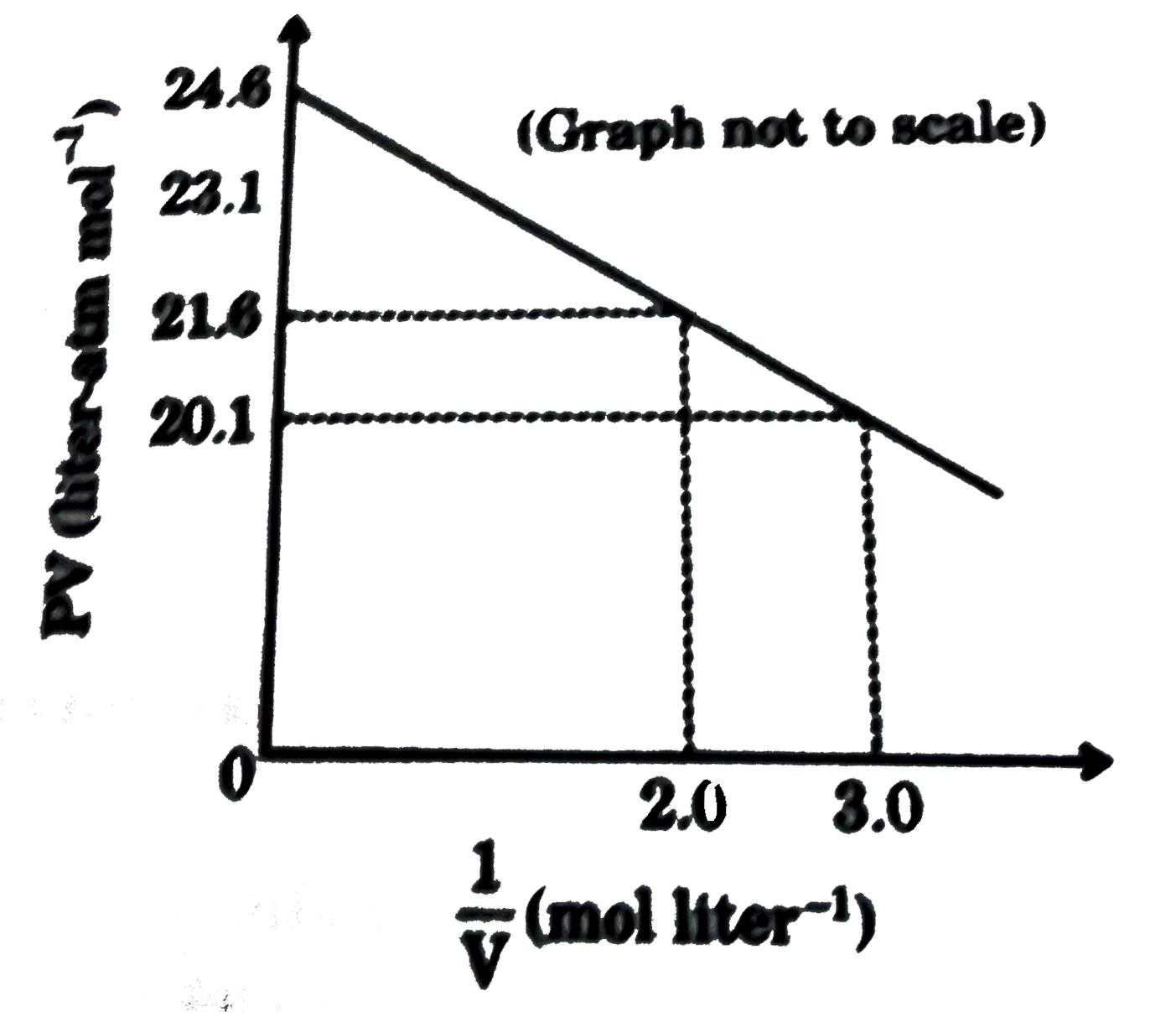
For one mole of a van der Waals' gas when b=0 and T=300K, the pV vs 1/

The compression factor compressibility factor for 1 mole of a van
- Derive an expression for the compression factor of a gas tha
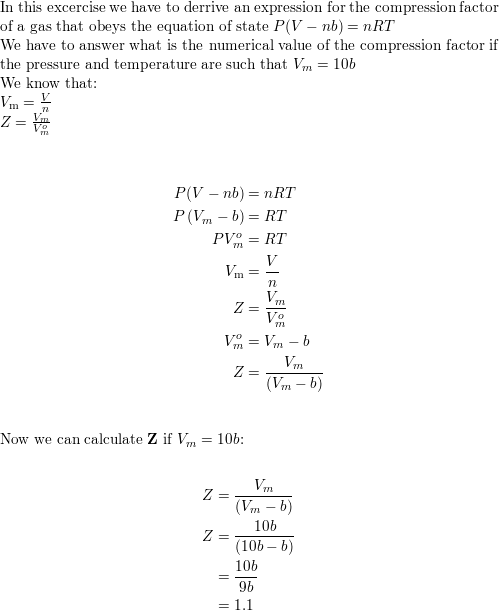
- the compression factor one mole of a vander waals gas 0 C and 100 atm pressure is found to be 0.5

- At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

- Compared with the graph of the parent function, which equation shows only a vertical compression by a

- Solved The compression factor (Z) for a real gas can be




