What is the compressibility factor (Z) for 0.02 mole of a van der

By A Mystery Man Writer

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0
SOLVED: 17. Calculate the compressibility factor for CO2, if one mole of it occupies 0.4 liter at 300 K and 40 atm. Comment on the result. (A) 0.40, CO2 is more compressible

Soil water diffusivity and water content distribution during outflow experiment

Compressibility Factor Calculator - File Exchange - MATLAB Central

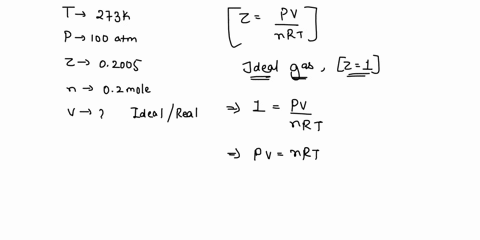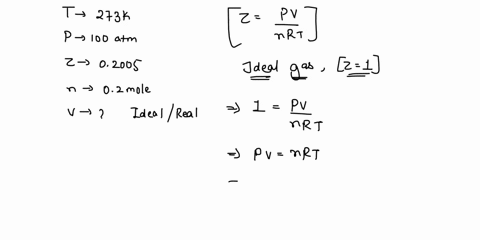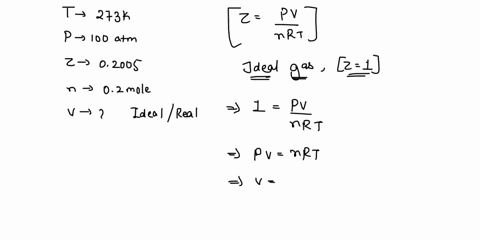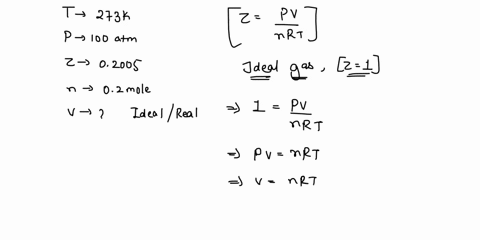
Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

compressible flow related terms - Department of Mechanical and

At `2173K` temp, and 9 atm pressure, the compressibility fog a gas is `0.9`. The volume of 1 mill-mo
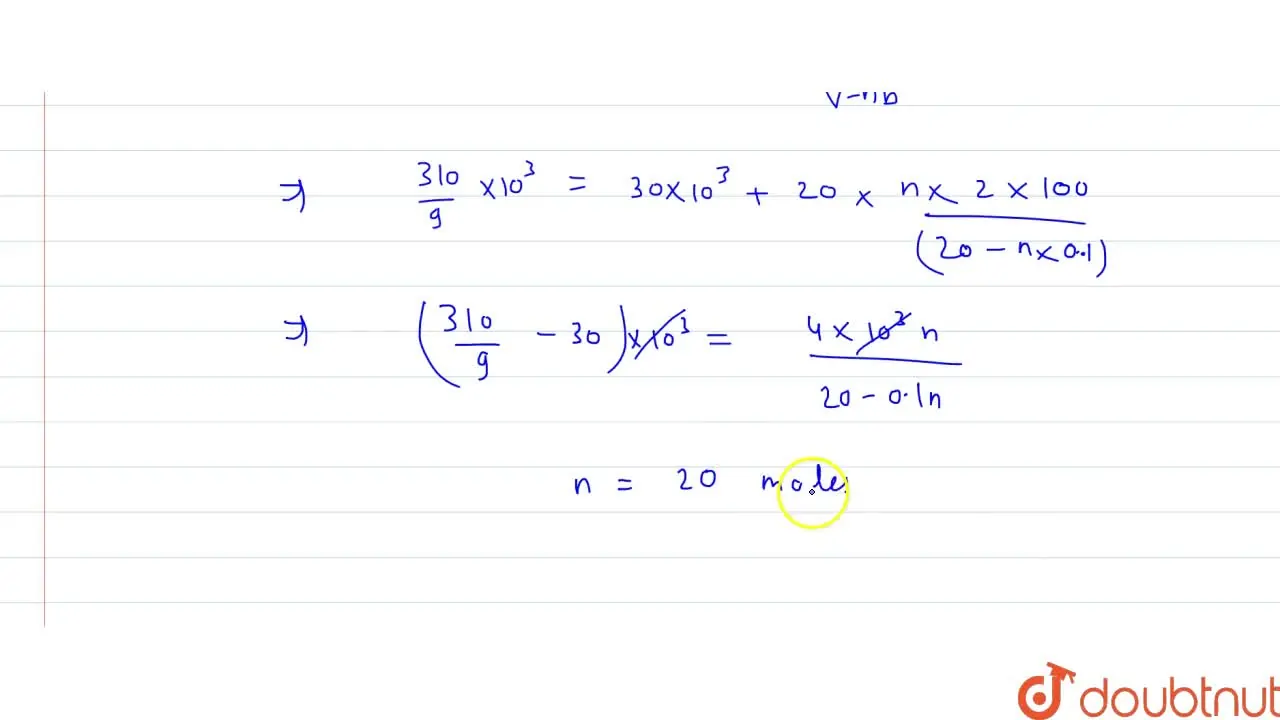
Some amount of diatomic van der walls' gas is kept in a rigid containe

Development of Graphical Methods for Estimating the Diffusivity Coefficient of Gases in Bitumen from Pressure-Decay Data

SOLVED: If PR=3, TR=2.0, the compressibility factor (Z) is equal to* 2 Points) a. Z=0.85 b. Z=0.55 c. Z=1.5 d. Z=0.95

Chemical Process Engineering - Harry Silla - Ventech!
- Standing and Katz gas compressibility factor
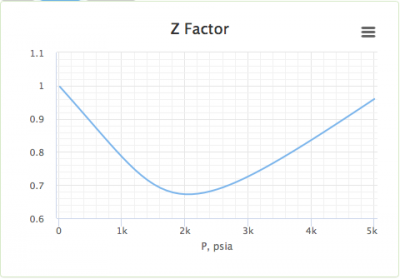
- Finding the compressibility factor (Z)
- Real Gases vs Ideal Gases & the Compressibility Factor
- Compressibility factor - Wikiwand

- In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar

- AMUL COMFY Women Hipster Multicolor Panty - Buy AMUL COMFY Women Hipster Multicolor Panty Online at Best Prices in India

- Starter pack: 2 boxers

- Free People - Intimately FP She's So Sleek Bodysuit in Ecru at Nordstrom

- Girl's (8-14) Side Line Sports Bra

- Large Ballerina Dancer Brass Statue, 1920s For Sale at 1stDibs

