Finding the compressibility factor (Z)
By A Mystery Man Writer

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Compressibility factor for methane.

SOLVED: 'Question 6 20 pts To find the specific volume of nitrogen

Compressibility Factor Z // Thermodynamics - Class 85
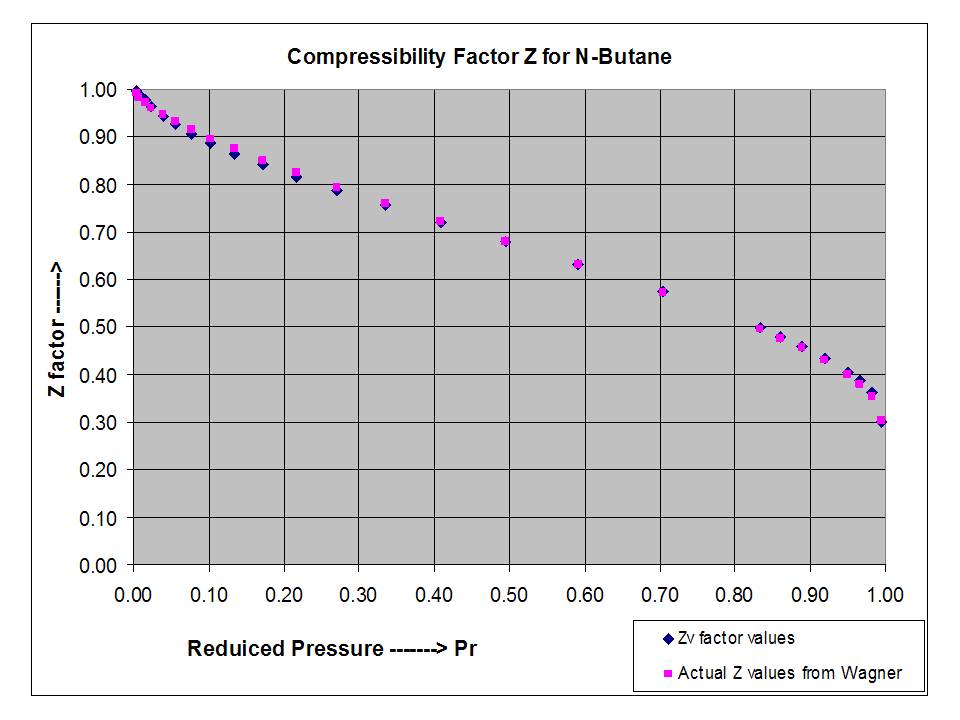
Two extensions of the compressibility factor Z correlation (sub-critical pressure region)
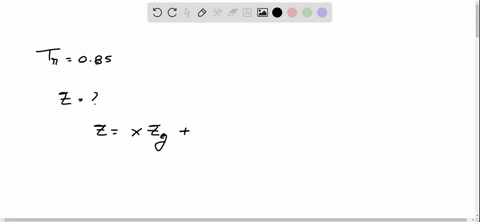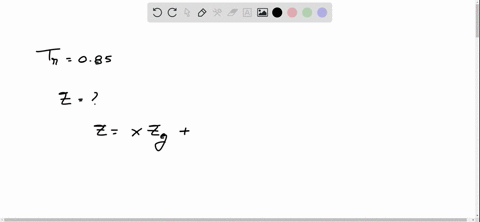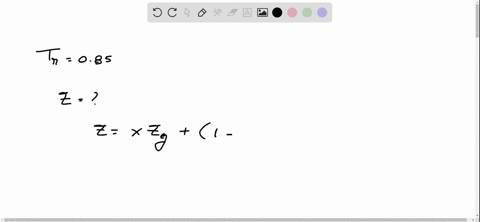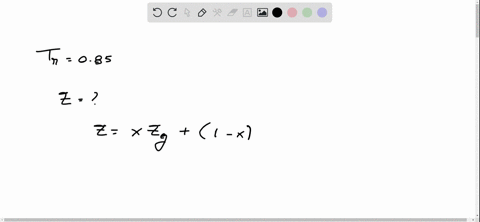
⏩SOLVED:With Tr=0.85 and a quality of 0.6 find the
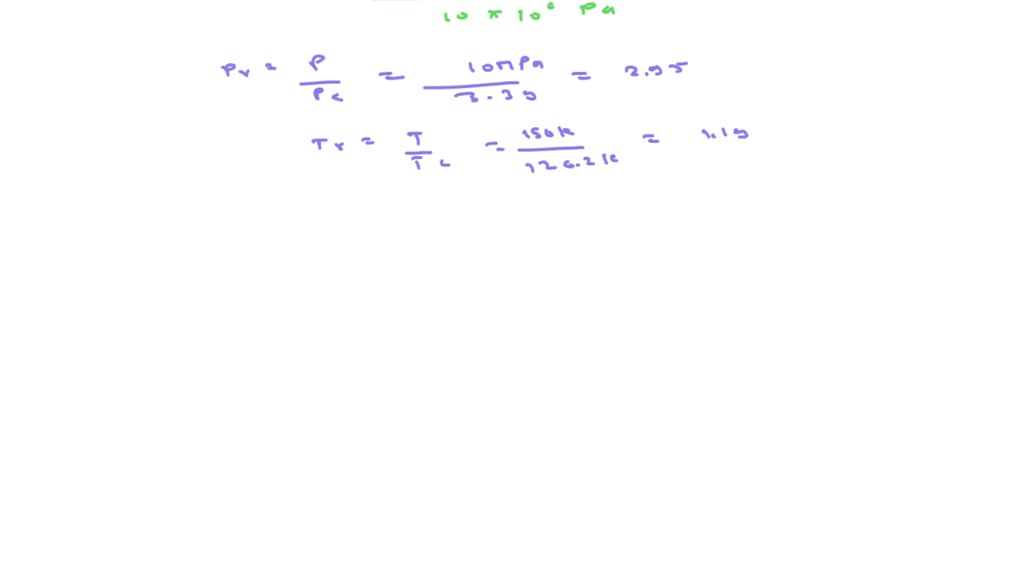
SOLVED: Determine the specific volume of nitrogen gas at 10 MPa

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

Super-critical Fluid Compressibility Factor Z , for Intermediate Reduced Pressure Range, a new correlation for excel spreadsheets

Gas Z Factor Calculator: Dranchuk-Abou-Kassem · PVT Solver

Compressibility factor (z): real gases deviate from ideal behav-Turito
- Compressibility factor for real gases

- Compressibility Factor Z for sub-critical pressures for Lee

- plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

- Super-critical Fluid Compressibility Factor Z , for Intermediate Reduced Pressure Range, a new correlation for excel spreadsheets

- In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar





