Solved] Why is the compressibility factor less than 1 at most conditions?
By A Mystery Man Writer

Gas Compressibility - an overview
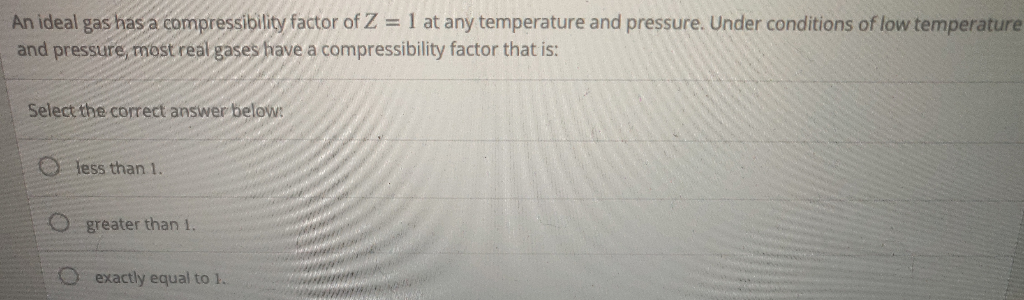
Solved An ideal gas has a compressibility factor of Z = 1 at

SOLVED: Problem 1: Answer the following concept-related questions: (a) Is it true that it takes more energy to vaporize 1 kg of saturated liquid water at 100°C than it would at 120°C? (

Compressibility Factor - an overview

Gas compressibility factor Z: Ideal gas vs Real gas

The compressibility factor of a gas is less than 1 at STP. Its mola
The value of compression factor at the critical state of a vander waals gas is
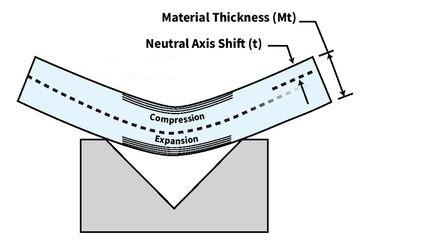
K-factors, Y-factors, and press brake bending precision

Compressibility factor (z): real gases deviate from ideal behav-Turito
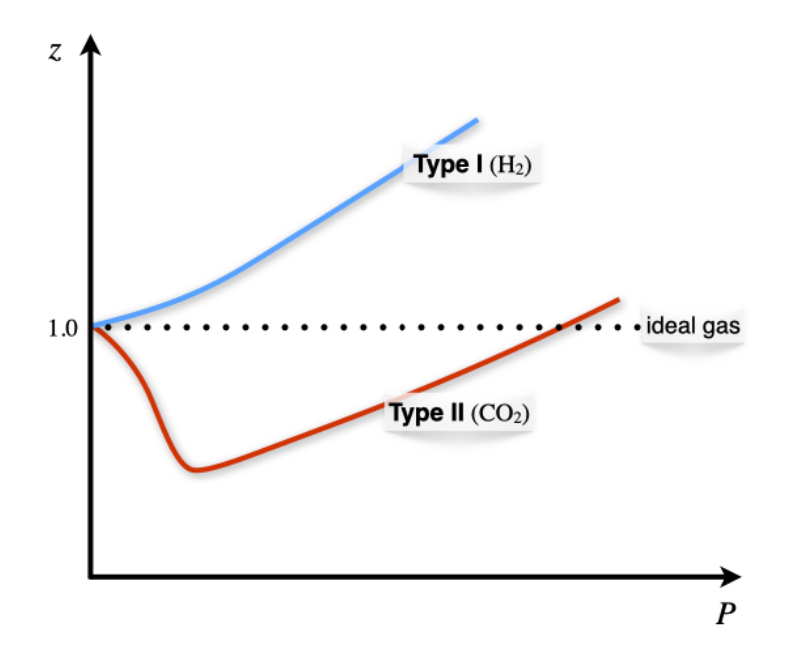
11.3: Critical Phenomena - Chemistry LibreTexts

Isothermal Compressibility. - an overview
- Compressibility factor - Wikipedia

- The value of compression factor at the critical state of a vander waals gas is
- Which of the following statements is/are correct? (a) all real gases are less compressible

- Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

- 000559 Calculation of Compressibility Factor from Redlich-Kwong





