Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

By A Mystery Man Writer
Results of the experimental measurements on the partial molar volume of kerosene used as a medium for dissolving TBP are utilized to determine the activity of TBP in the binary kerosene-TBP solution through the application of Gibbs-Duhem equation. The treatment is based on combination of the experimental data with the thermodynamic values available on the compressibility factor of pure kerosene at room temperature. It is shown that the activity of TBP in kerosene has a positive deviation from ideality with an activity coefficient derived as follows:1) at X TBP ≤ 0.01: γ TBP = 42.530, 2) at the 0.01 X TBP 0.2: 3) at the higher TBP concentrations 0.2 X TBP 0.97: and 4) at TBP Raoultian concentrations 0.97 ≤ X TBP:γ TBP = 1. These quantities can be utilized at temperature closed to 298 K.

Water adsorption in the organic phase for the D2EHPA-kerosene

Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

Nuclear Energy and Environmental Impact

PDF] EXTRACTION OF ZN, MN AND CO FROM ZN-MN-CO-CD-NI CONTAINING

Synergistic effect of MEHPA on co-extraction of zinc and cadmium

Solvent Extraction of Nickel and Zinc from Nitric Acid Solution

Developing a Thermodynamical Method for Prediction of Activity Coefficient of TBP Dissolved in Kerosene

Chinese Journal of Chemical Engineering

E. ALAMDARI, Professor (Associate), PhD

Extraction equilibrium of molybdenum(VI) and tungsten(VI) in aqueous solutions containing hydrogen peroxide by synergistic solvent extraction with TRPO and TBP - ScienceDirect

Developing a Thermodynamical Method for Prediction of Activity

PDF] EXTRACTION OF ZN, MN AND CO FROM ZN-MN-CO-CD-NI CONTAINING
- Solved Show that the compressibility factor of van der Waals

- The value of compression factor at the critical state of a vander waals gas is
- If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

- 1. The compressibility factor, z, is the ratio of

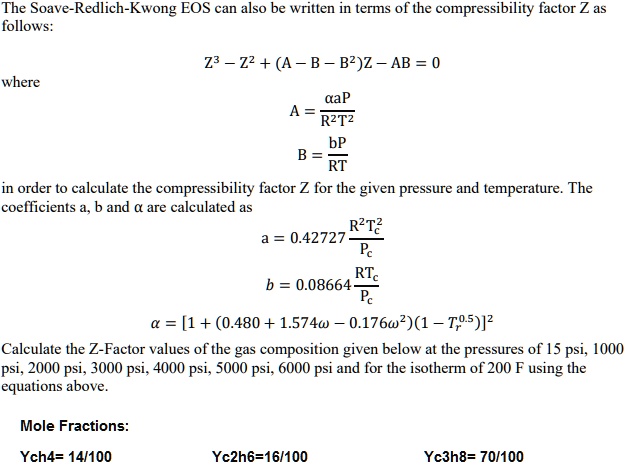
- SOLVED: The Soave-Redlich-Kwong EOS can also be written in terms of the compressibility factor Z as follows: Z^3 - Z^2 + (A - B - B^2Z - AB) = 0 where A =
- Cacique, Intimates & Sleepwear, Cacique Smooth Lightly Lined Balconette Bra Red Pink Lace 42f

- Andrew Scott Kids Boys Girls Toddler Fleece Brush Lined Stretch Leggings

- Kalyan Silks - Al Fahidi (Clothing) in Bur Dubai Get Contact Number, Address, Reviews, Rating - Dubai Local

- I felt ashamed of my DDD boobs at work & struggled to find sexy

- Mammoth Ivory Hooded Woman Cameo Jewelry [67911] Large
