The value of compression factor at the critical state of a vander waals gas is
By A Mystery Man Writer
The value of compression factor at the critical state of a vander waals gas is
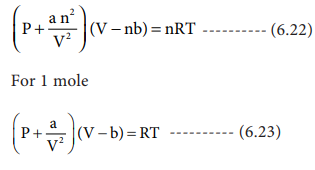
Derivation of critical constants from van der Waals constant

Gaseous State.pdf - Chemistry - Notes - Teachmint

What is the value of z (compressibility factor) for a vander waal gas at critical
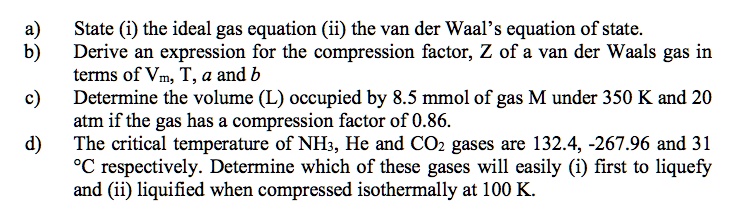
SOLVED: State (i) the ideal gas equation (ii) the van der Waal's equation of state. Derive an expression for the compression factor; Z of a van der Waals gas in terms of

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application

Non-ideal behavior of gases (article)
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
Why there is different between the value of compressibility factor at critical point between real and ideal gas? - Quora

Fluids, Free Full-Text

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is

Compressibility factor under critical state of a gas is :dfrac{5}{8}dfrac{8}{3}dfrac{3}{8R}dfrac{3}{8}
- Sport-Tek® T474 Dri-Mesh® Pro Polo

- Trauma and First Aid Kits (TFAK) - Class A
- Plus Size Period Leak Proof Undies Heavy, Sizes 12-30

- 5 signs it's time to see a physical therapist - Northeast Georgia

- Women's Garter Lingerie, Sexy Lace Lingerie, Sheer Matching 4-Piece Lingerie Sexy Underwire Gathering Bundle Split Sexy Lingerie - AliExpress





