At high pressure, the compressibility factor 'Z' is equal toa

By A Mystery Man Writer

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts

PDF) Natural Gas Compressibility Factor Measurement and Evaluation for High Pressure High Temperature Gas Reservoirs

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts

6.3: Van der Waals and Other Gases - Physics LibreTexts
At very high pressure, the compressibility factor of one mole of a gas

SOLVED: Hey guys, please help me friends. Choose the correct answer, don't say wrong answers. Select correct statement/s regarding compressibility factor Z of a gas: Z for an ideal gas is independent

At high pressure, the compressibility factor 'Z' is equal toa
At very high pressure, the compressibility factor of one mole of a gas
- Excel Calculations: Compressibility Factor for Natural Gas
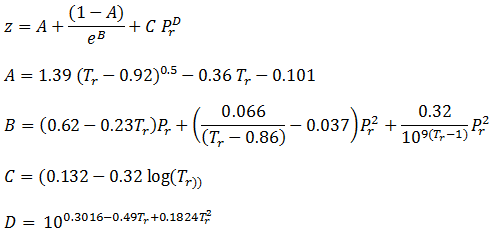
- If Z is a compressibility factor, van der Waals equation at low pressure ..

- Solved] Why is the compressibility factor less than 1 at most conditions?
- Compressibility factor (gases) - Knowino

- ChemE 260 Equations of State April 4, 2005 Dr. William Baratuci





