UNUB At Boyle temperature, the value of compressi factor Z has a value of one over a wide range of pressure. This is due to the fact that in the van der

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

economy Archives - Brazilian-American Chamber of Commerce

PDF) The Total Image Process * Alternative Sight Vision Transducer
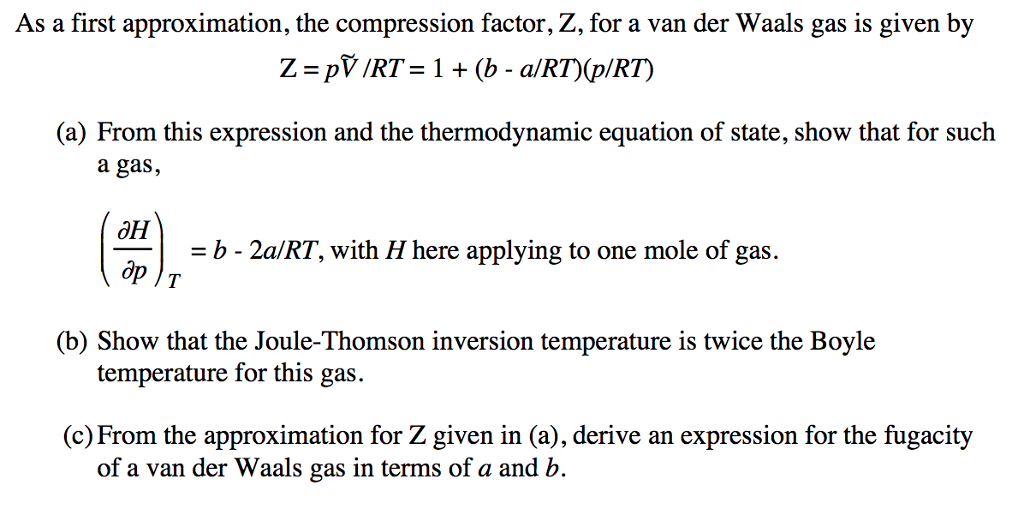
Solved As a first approximation, the compression factor, Z
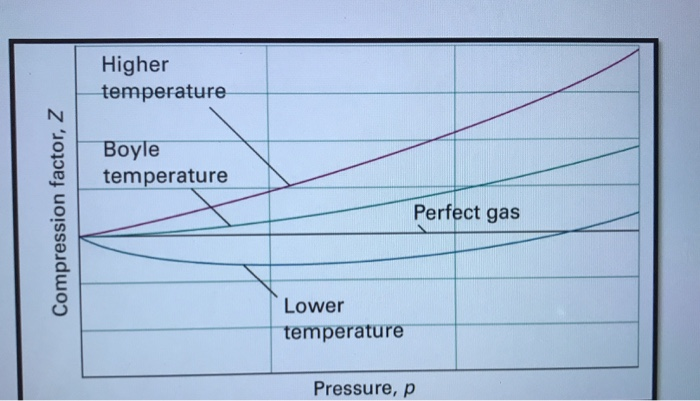
Solved 6. (a) Discuss the significance of the Boyle
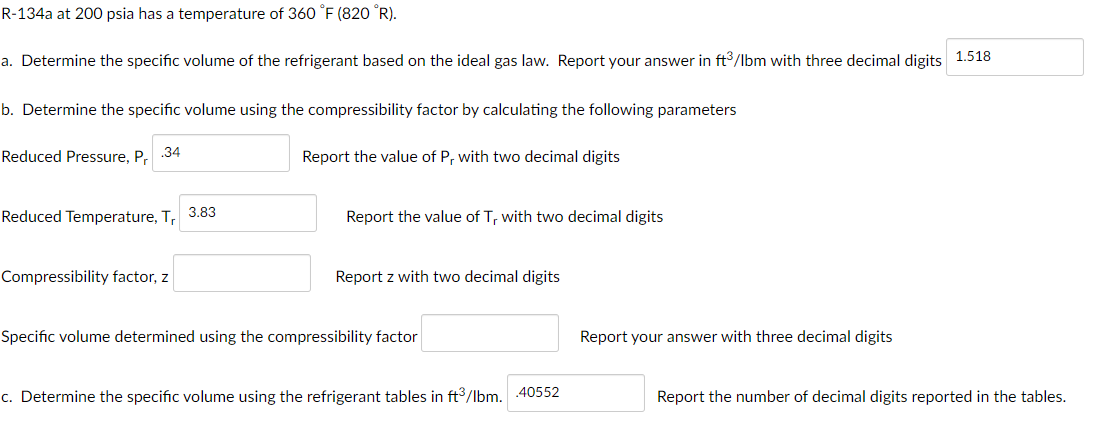
Solved b. Determine the specific volume using the
The value of compression factor at the critical state of a vander
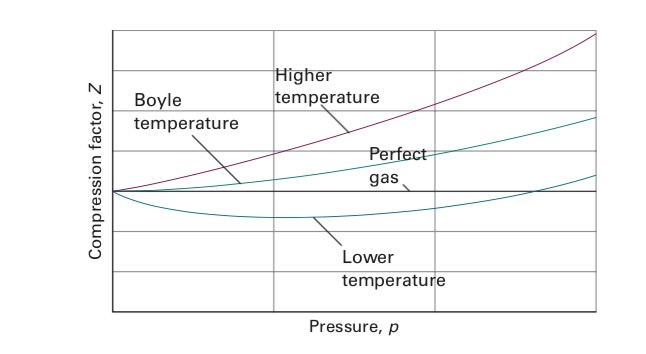
Solved I have a question about Boyle Temperature. I
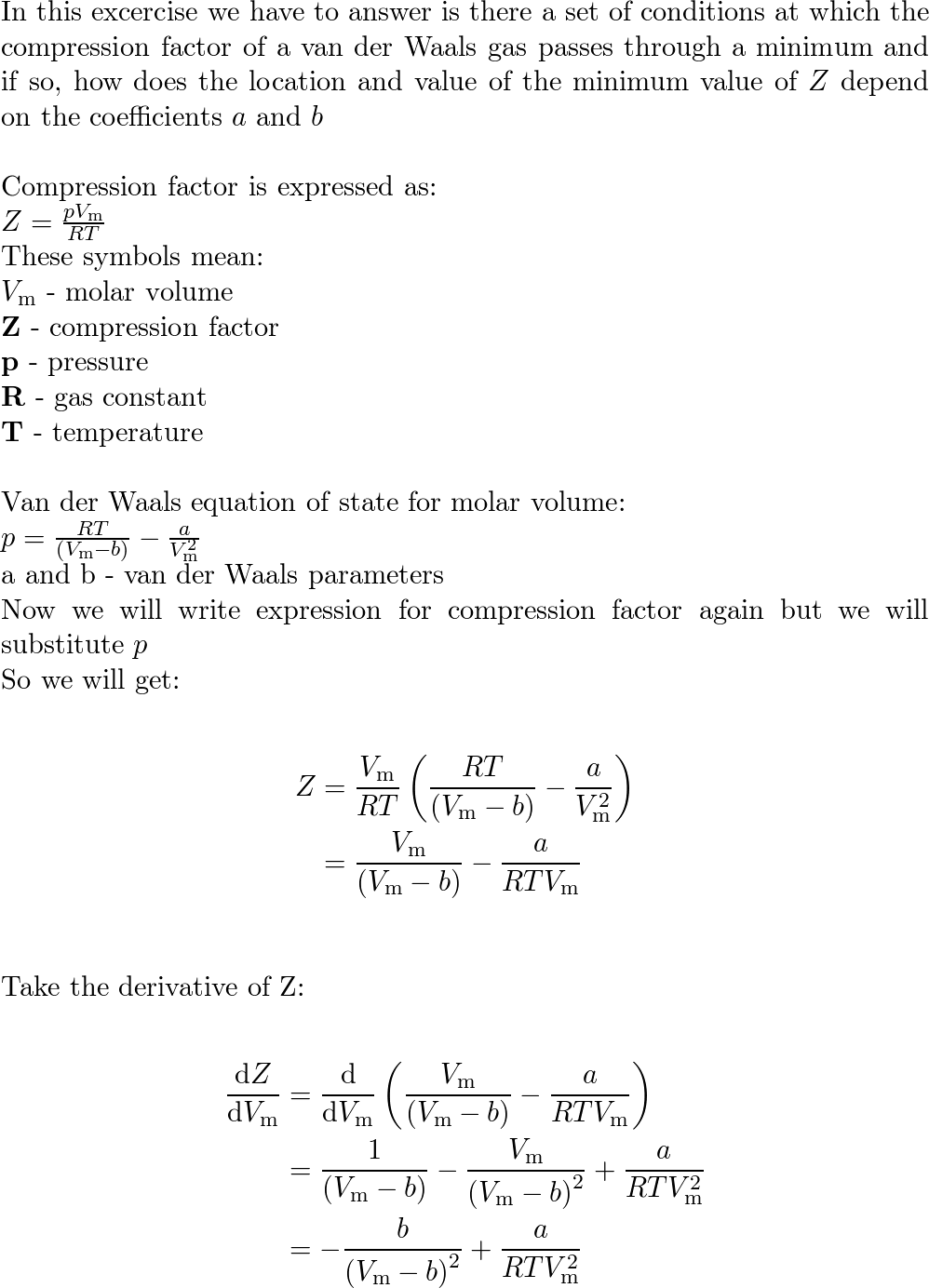
Is there a set of conditions at which the compression factor
At Critical Temperature,pressure and volume . The compressibility

Below the boyle temperature ,explain the effect of temperature on

A LEVEL Heat and Modern 2016, PDF, Thermometer

Respostas - Físico-Química (Vol.1) - Atkins PDF
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
- At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{

- Real Gases. The ideal gas equation of state is not sufficient to

- SOLVED: For a gas at a given temperature, the compression factor

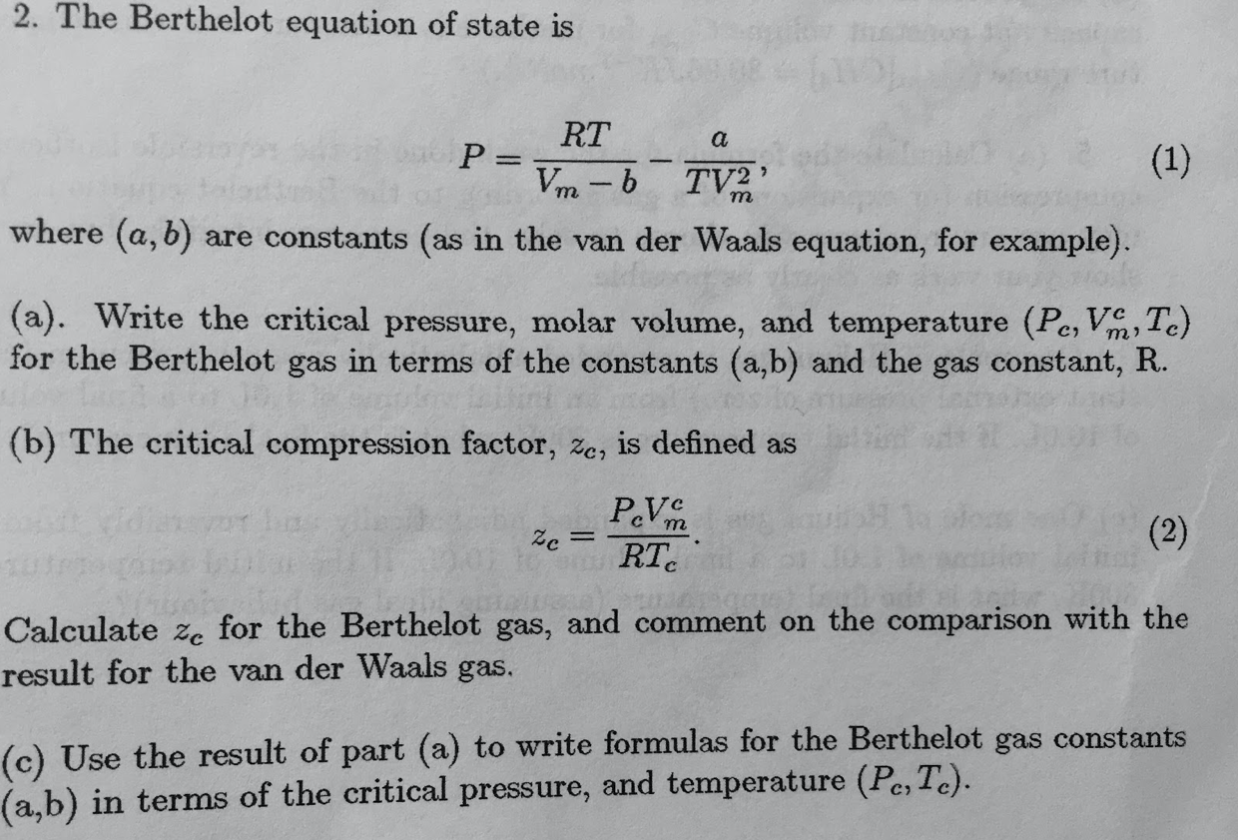
- Solved 2. The Berthelot equation of state is (1) RT P= Vm
- TERRA & SKY PLUS SCOOP NECK TS RIB LAYERING TANK 3X (24w-26w) NEW / TAG SKU A21 $8.00 - PicClick

- Tek Gear 's Black Pants for Women for sale

- I have bigger boobs and it's such a pain - most tops only look good on women with fake perky breasts

- Spring Sale Banner Template Graphic by captoro · Creative Fabrica

- Charmed Triquetra Cat Collar
