At 273 K measurements on argon gave B = -21.7 cm$^3$ mol$^{

By A Mystery Man Writer

Accurate (p, ρ, T, x) Measurements of Hydrogen-Enriched Natural-Gas Mixtures at T = (273.15, 283.15, and 293.15) K with Pressures up to 8 MPa

1.2 The kinetic model of gases
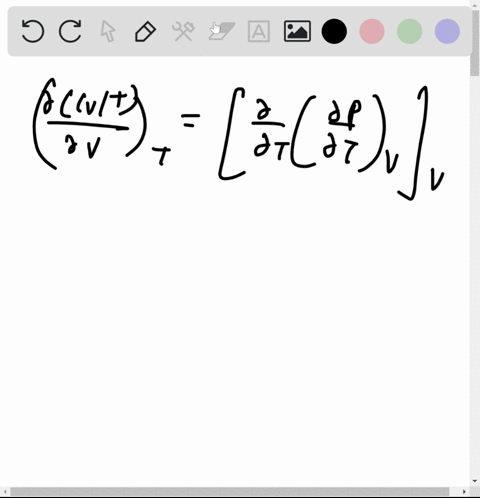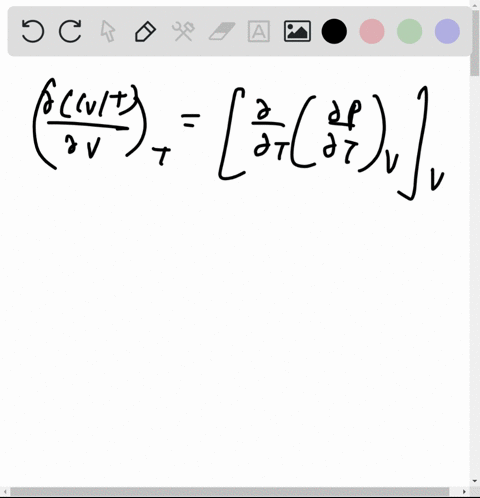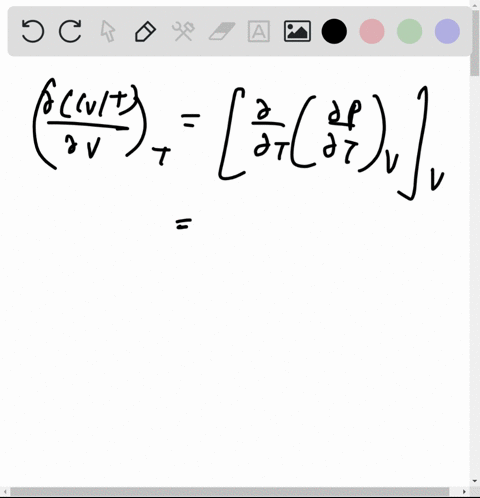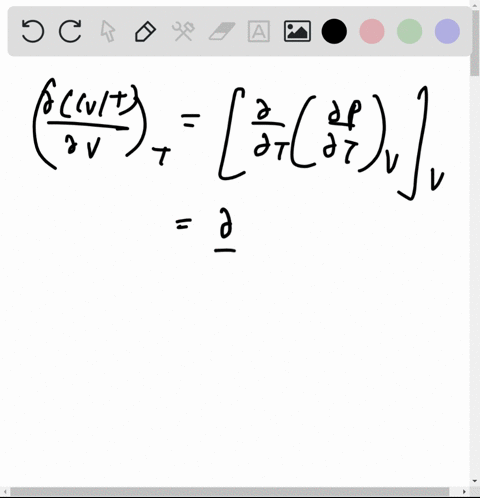
⏩SOLVED:At 273 K measurements on argon gave B=-21.7 cm^3 mol^-1 and…

Forecasting global and multi-level thermospheric neutral density and ionospheric electron content by tuning models against satellite-based accelerometer measurements
How does one find out the volume of a gas at STP? - Quora

Measuring Gas Pressure - Chemistry
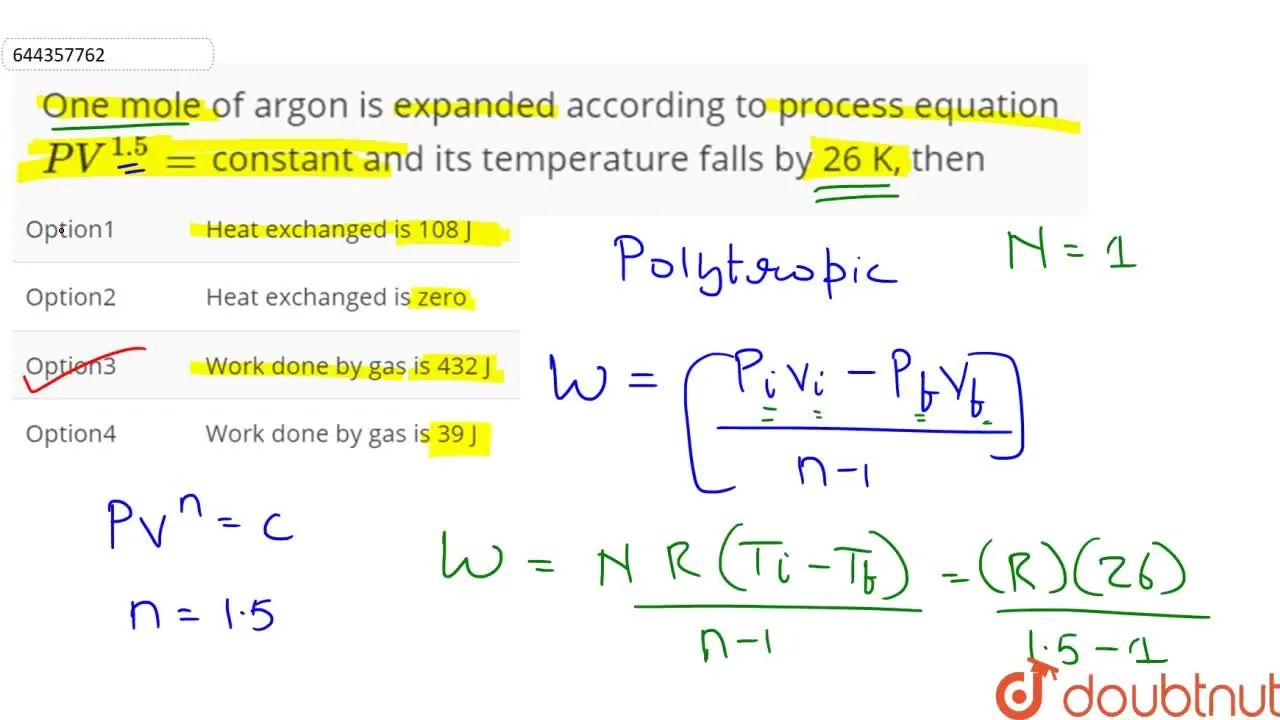
One mole of argon is expanded according to process equation PV^(1.5)=c
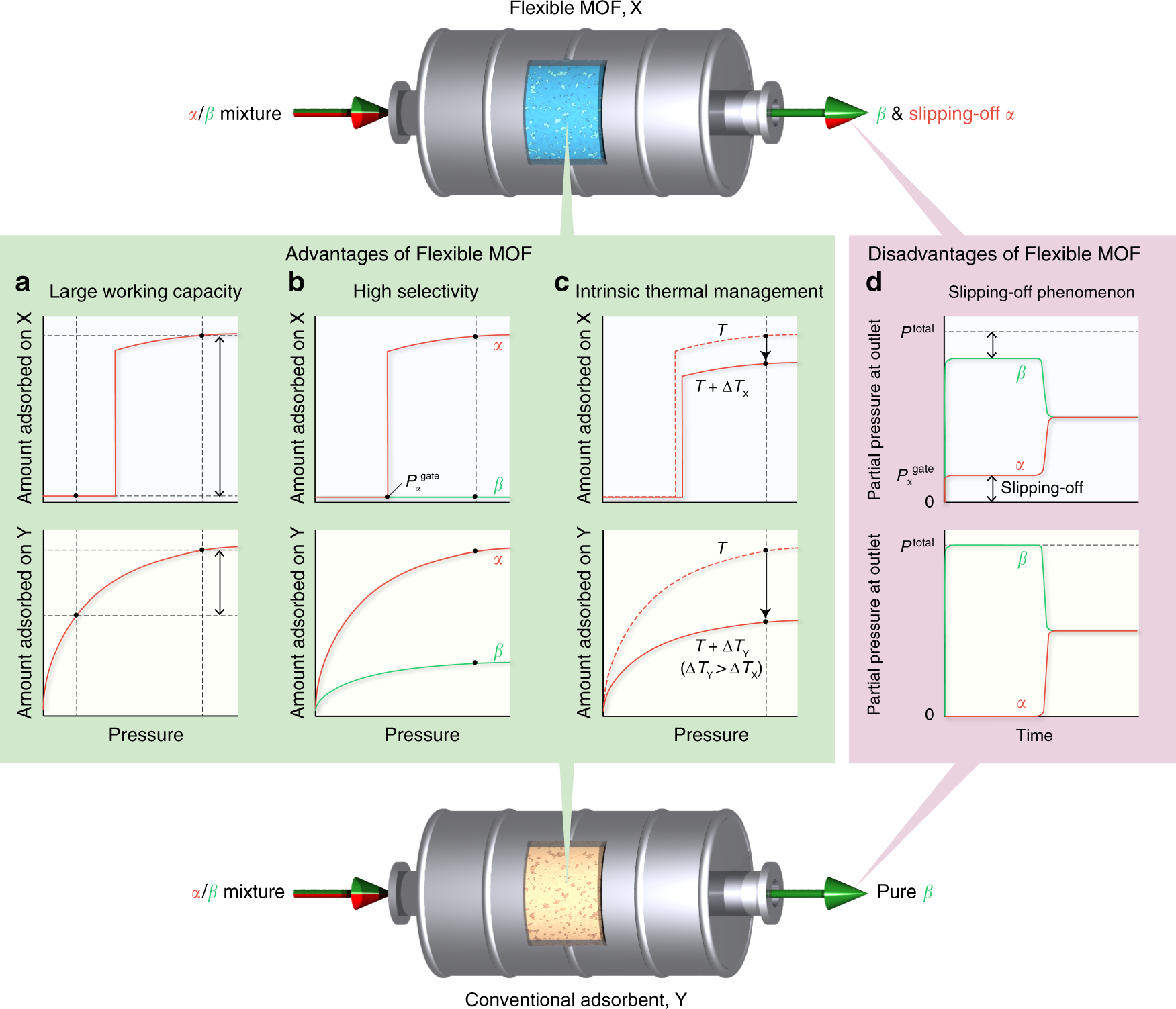
High-throughput gas separation by flexible metal–organic frameworks with fast gating and thermal management capabilities

One mole of argon is expanded according to process equation PV^(1.5)=c
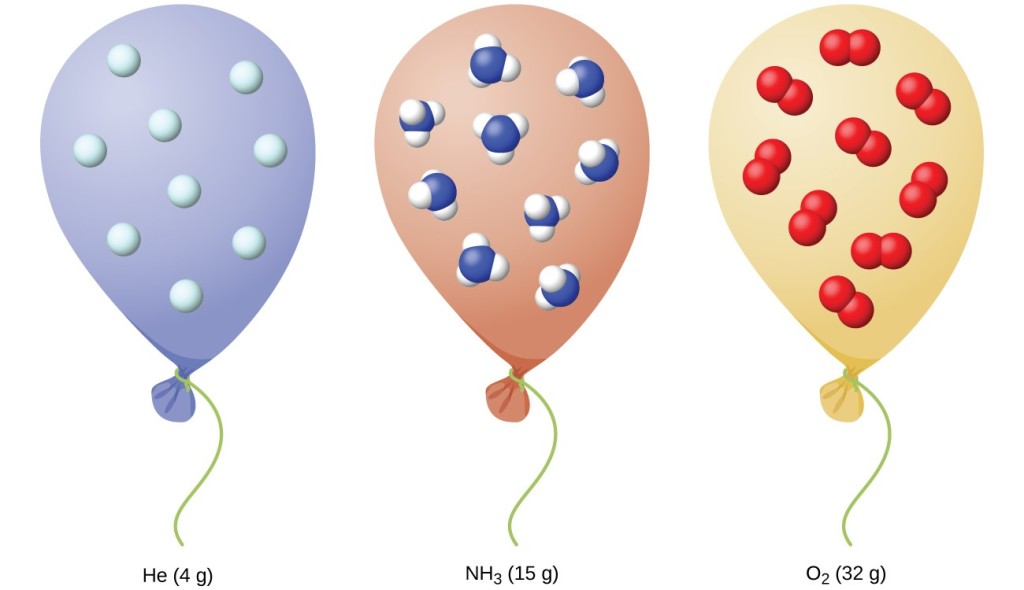
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
-2.png)
Solved] The following financial statements apply
- Compression Factor Z

- Solved 6. Let's see if we can derive an alternative
- 53 pts!! The function f(x)= 7^x+1 is transformed to function g through a horizontal compression by a factor

- If `Z` is a compressibility factor, van der Waals' equation at low

- Find the isothermal compressibility `x` of a Van der Walls gas as






