Monday, Jul 08 2024
What is the change in internal energy (in J) of a system that absorbs 0.464 kJ of heat from its surroundings and has 0.630 kcal of work done on it?

By A Mystery Man Writer
I found an increase of 3100J Have a look

Solved What is the change in internal energy in J) of a

Section 4
Solved What is the change in internal energy (in J) of a
53. During a process a system absorbs 710 J of heat and does work

How to calculate ΔE when the system absorbs 250 J of heat energy

Energy: Production, Conversion, Storage, Conservation, And

A system absorbs 10 kJ of heat and does 4 kJ of work the internal
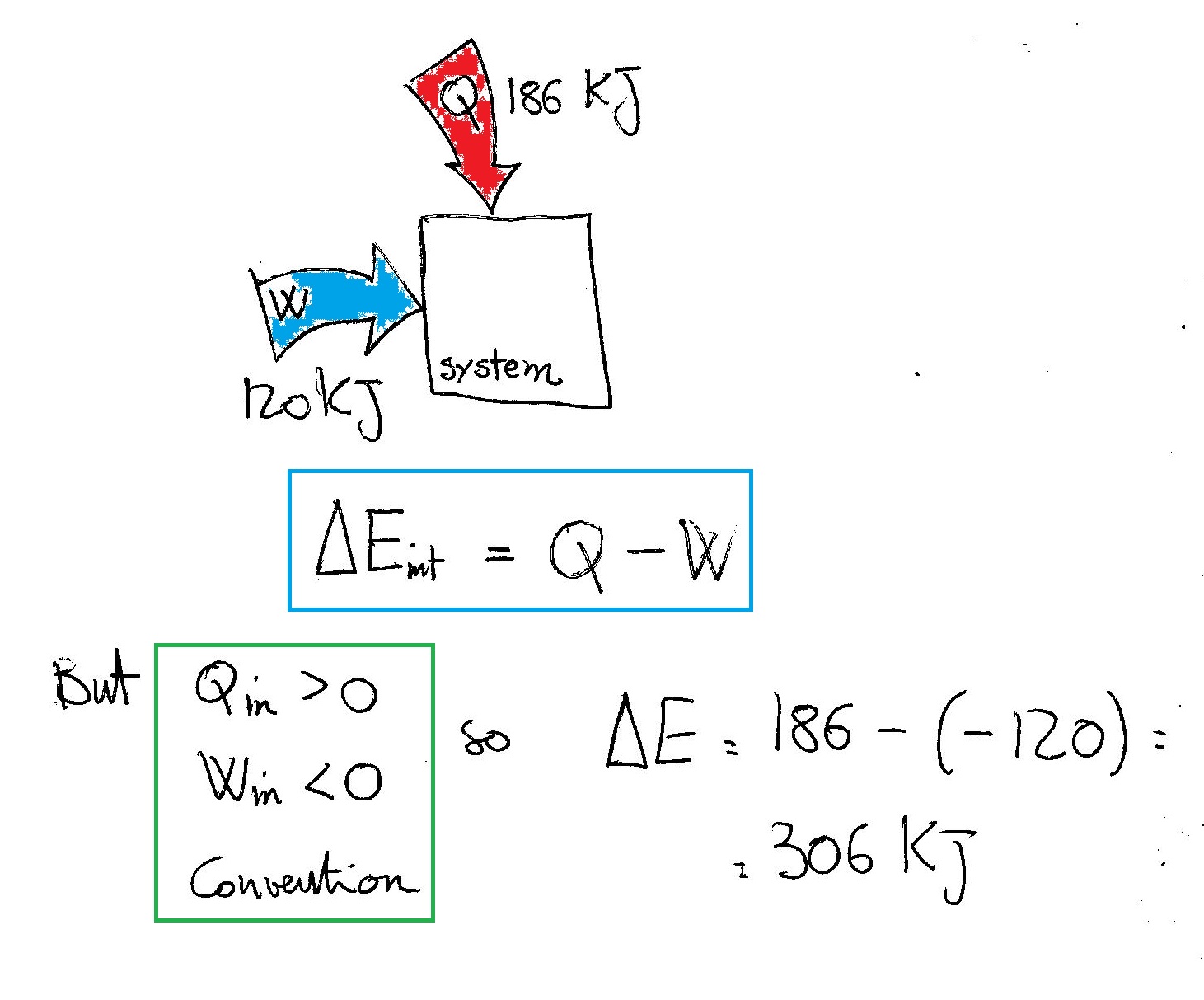
A system absorbs 186 kJ of heat and the surroundings do 120 kJ of

What is the change in internal energy (in J) of a system tha

Section 4

Appendix CA: Modified National Standard for Buildings, Except for
Related searches
- What is an Absorber? Answered by Twinkl - Twinkl
- Scientists discover ultra-black fish that absorbs 99.5% of light that hits its skin

- Absorption and Emission — Definition & Overview - Expii

- Chapter 10. Cell and Tissue Architecture: Cytoskeleton, Cell Junctions, and Extracellular Matrix
- What is the change in internal energy (in J) of a system that

©2016-2024, doctommy.com, Inc. or its affiliates




