What is the change in internal energy (in J) of a system that

By A Mystery Man Writer
I found an increase of 3100J Have a look
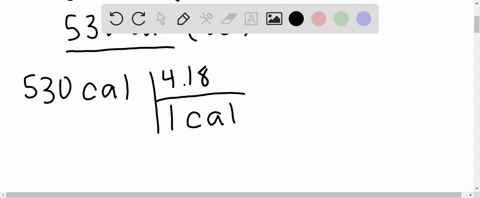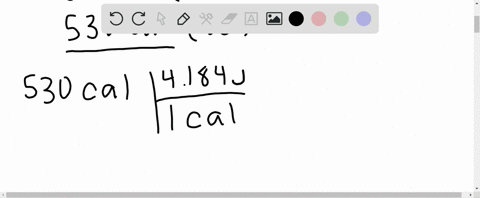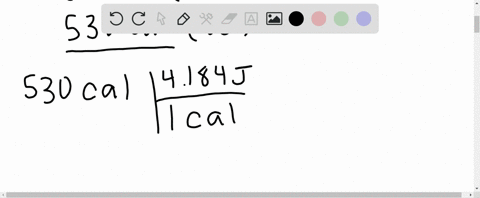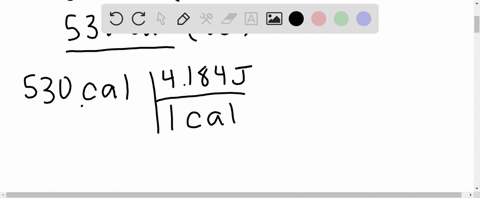
⏩SOLVED:What is the change in internal energy (in J) of a system

A system conducts 255 cal of heat to the surroundings while

SOLVED: 1.3) A system releases 125 kJ of heat while 104 KJ of work

Ch6.1 The Nature of Energy (hustle!) - ppt download

The internal energy change in a system that has absorbed 2 kcals of heat - NEETLab

Answered: What is the change in internal energy…

15.4 What is the change in internal energy of a system which
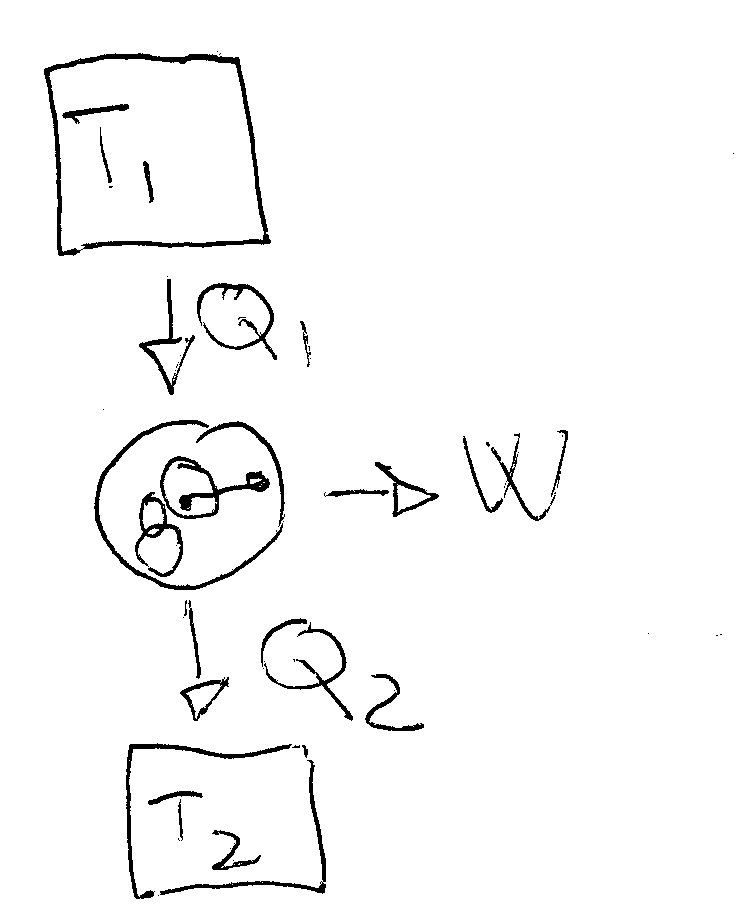
Heat and Work - Physics

500 J of heat was supplied to a system constant volume. It resulted in the increase of temperature of the system from 20^oC to 25^oC. What is the change in internal energy
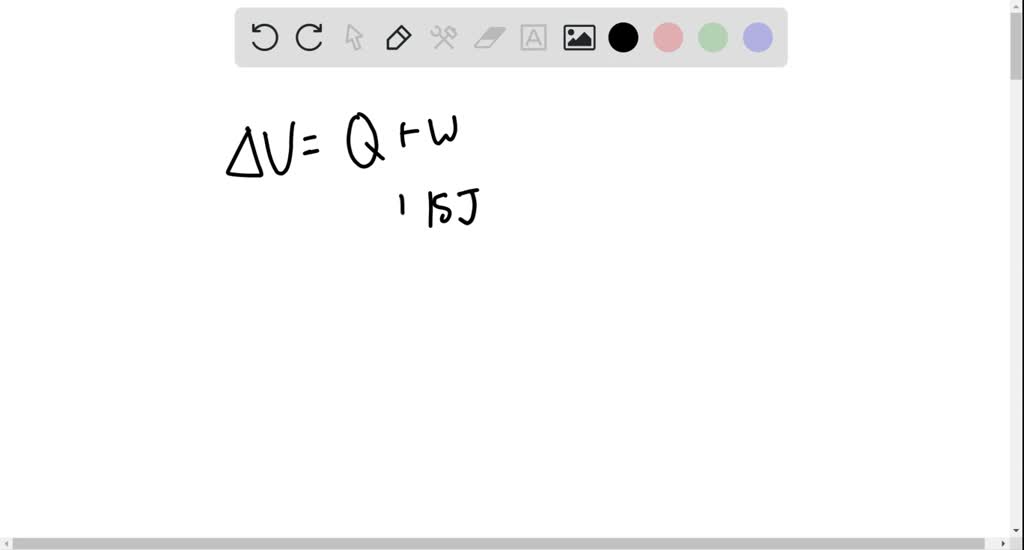
⏩SOLVED:What is the change in the internal energy of a system if
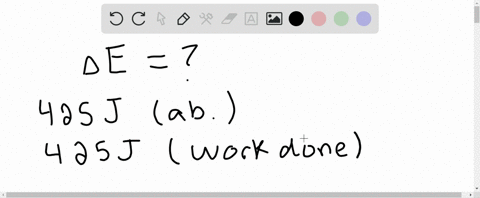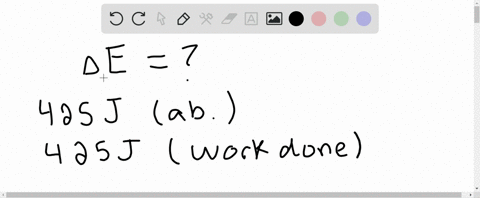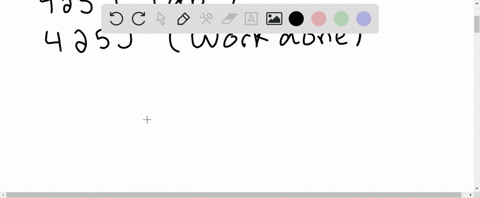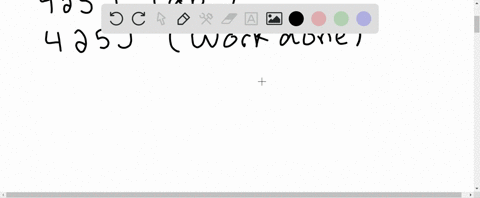
⏩SOLVED:A system receives 425 J of heat from and delivers 425 J

SOLVED: What is the change in internal energy of a system if the

Answered: What is the change in internal energy…
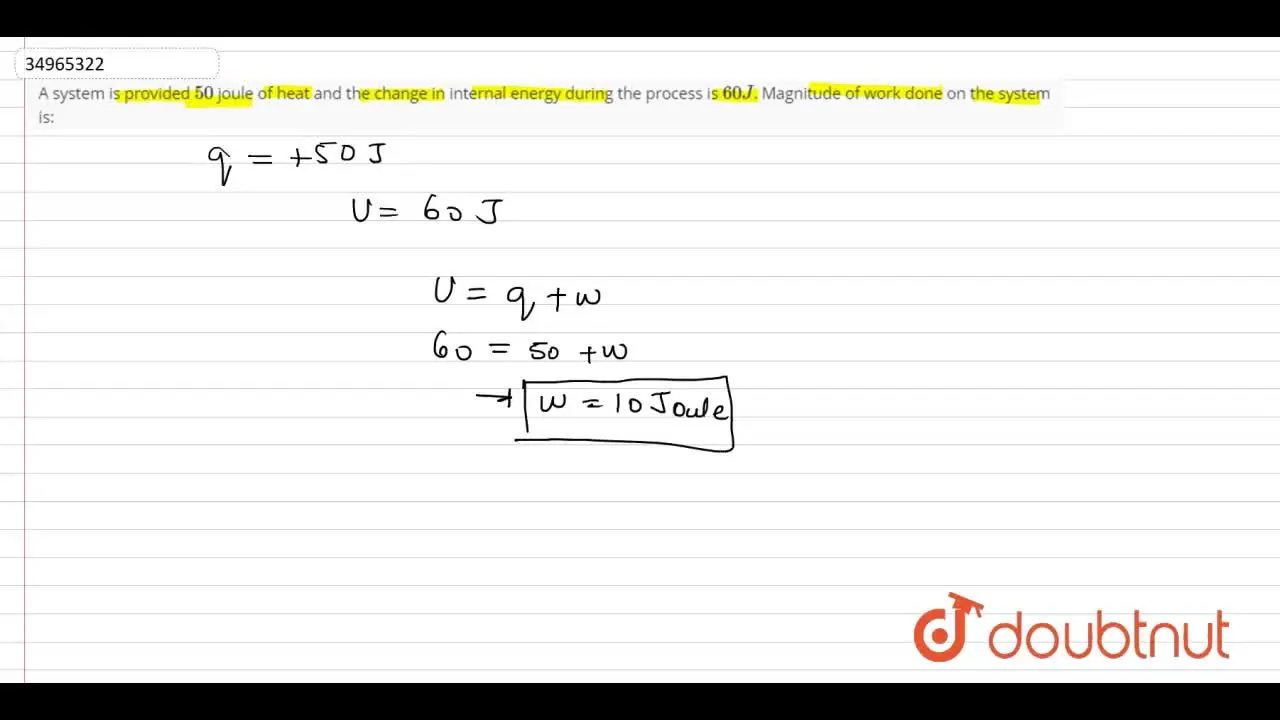
A system is provided 50 joule of heat and the change in internal energ
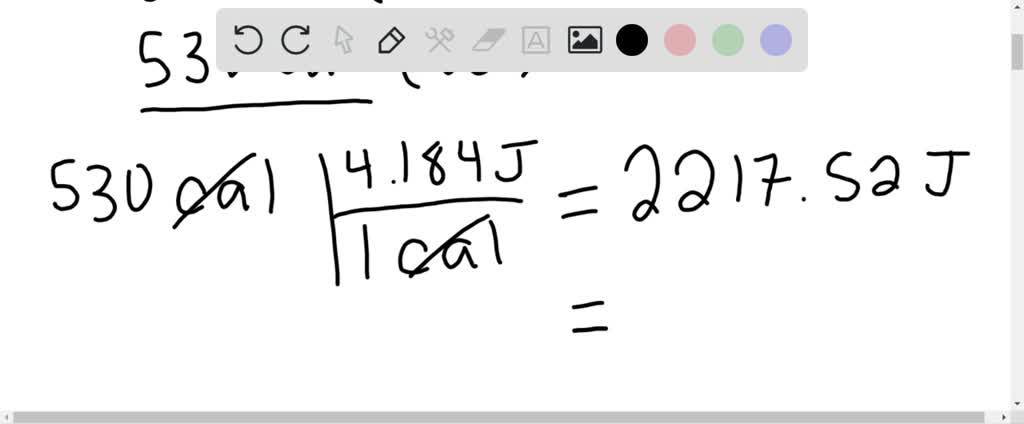
⏩SOLVED:What is the change in internal energy (in J) of a system