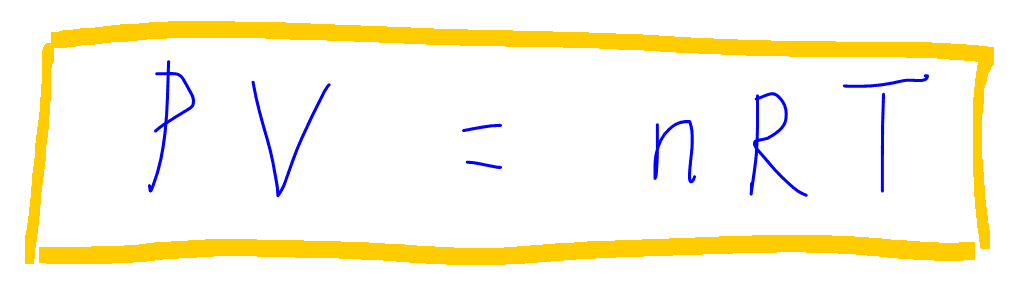
Solved An ideal gas initially at Pi, Vi, and Ti is taken
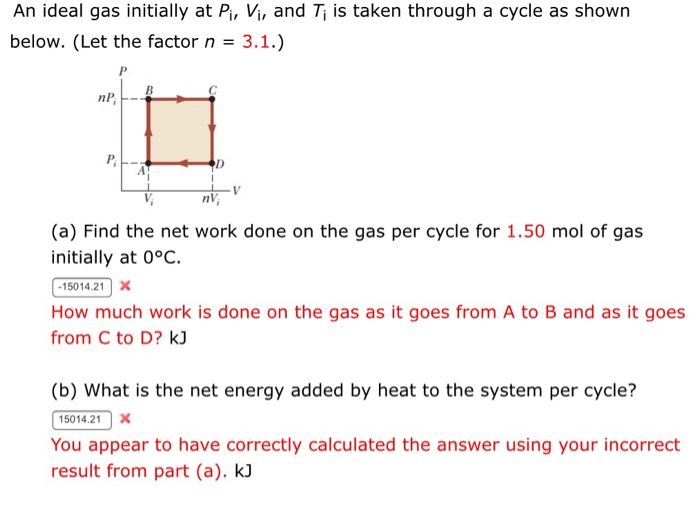
By A Mystery Man Writer
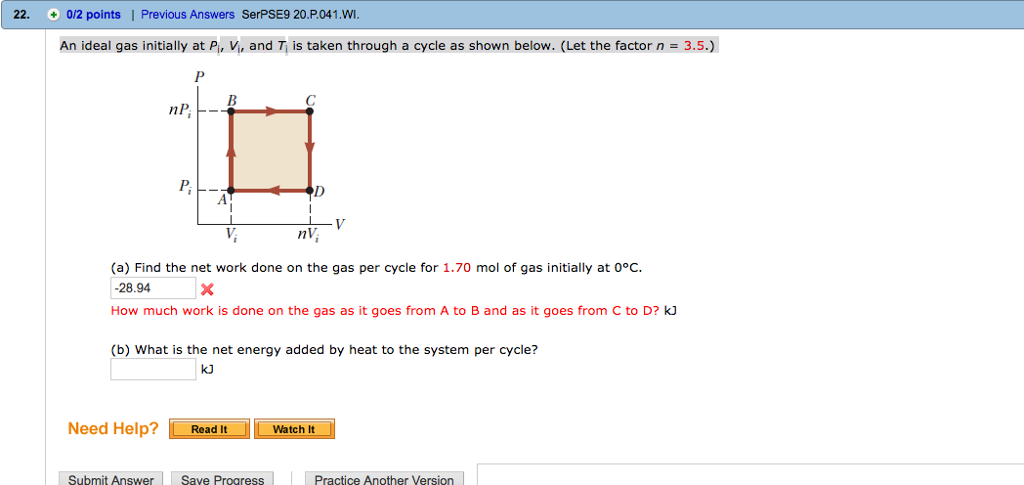
Solved An ideal gas initially at Pi, Vi, and Ti is taken

Boyle's Law — Overview & Formula - Expii

mohol an Ideal gas al 300 K occupies a volume of 0.36 m of 2 atm. The gas expands adiabatically its volume becomes 144. Net gas is compressed isobarically to its original
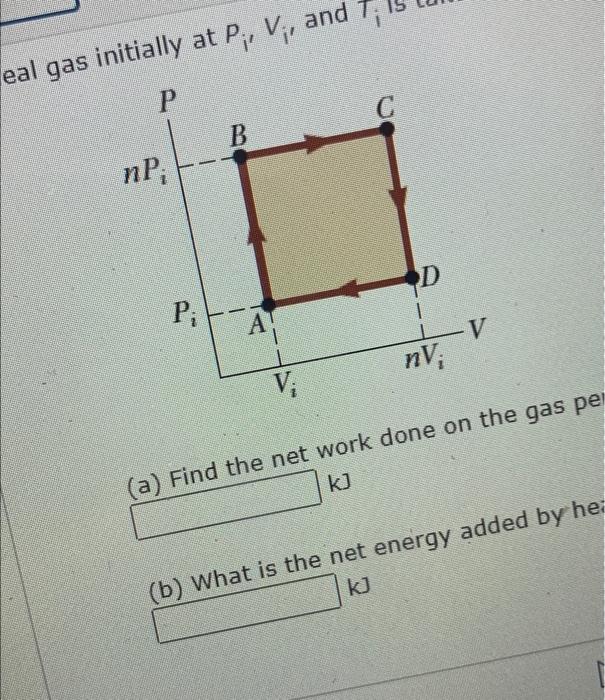
Solved An ideal gas initially at Pi, Vi, and Ti is taken
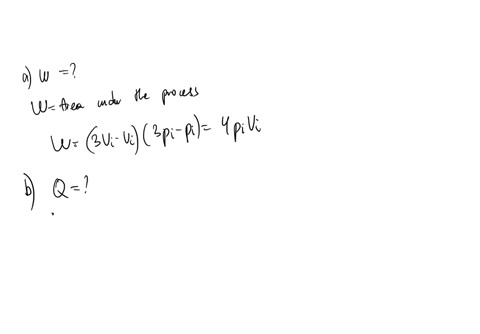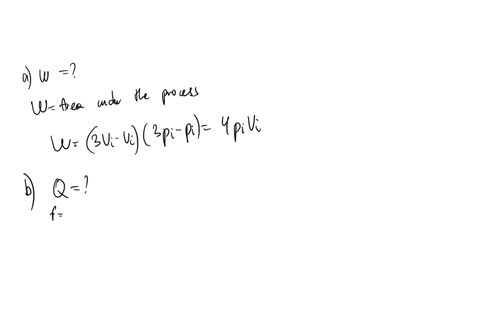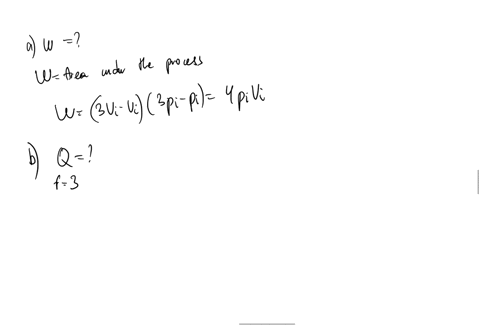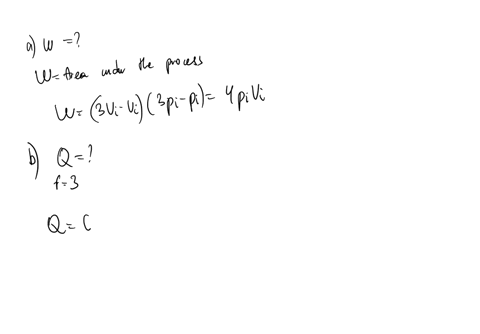
SOLVED: An ideal gas initially at Pi' Vi' and Ti is taken through a cycle as shown below. (Let the factor n 2.8.) nP; nV; a) Find the net work done on
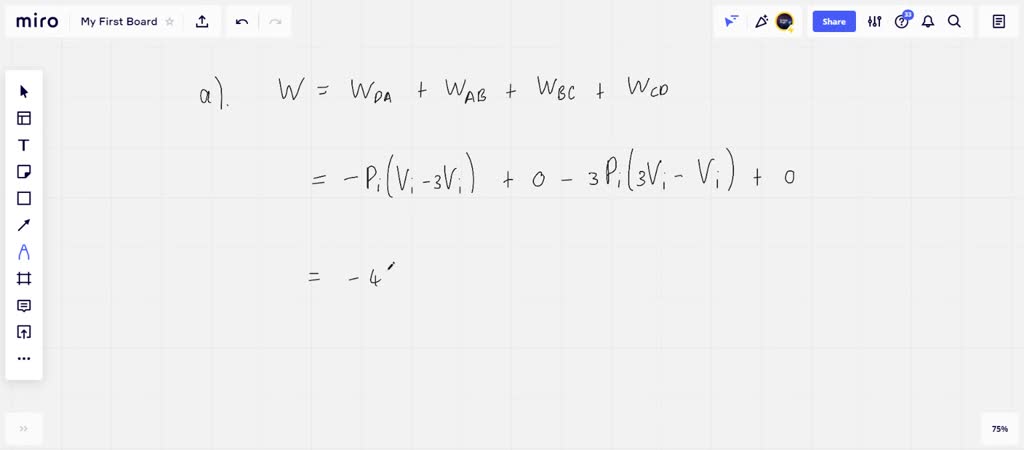
SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…
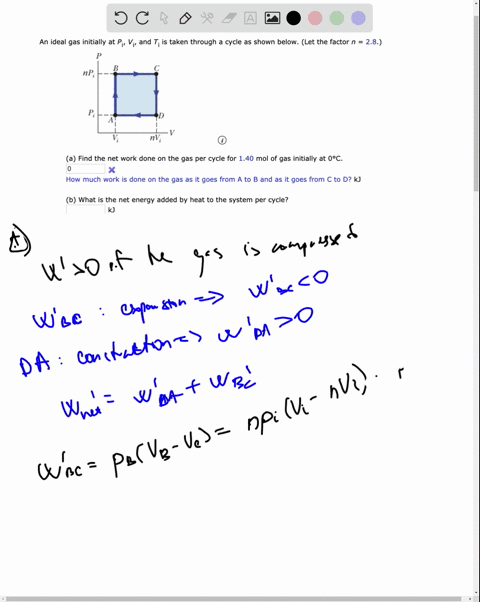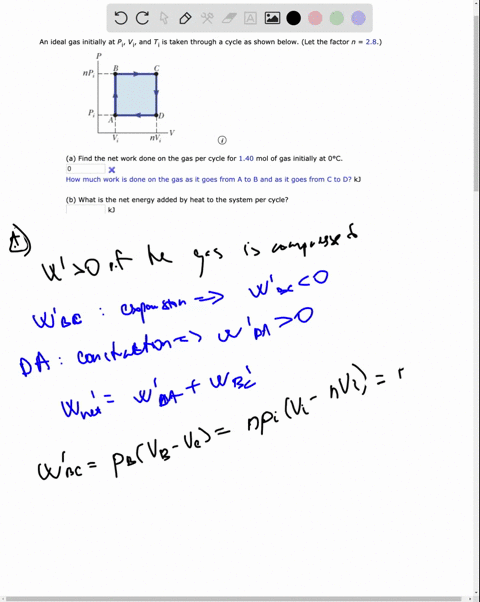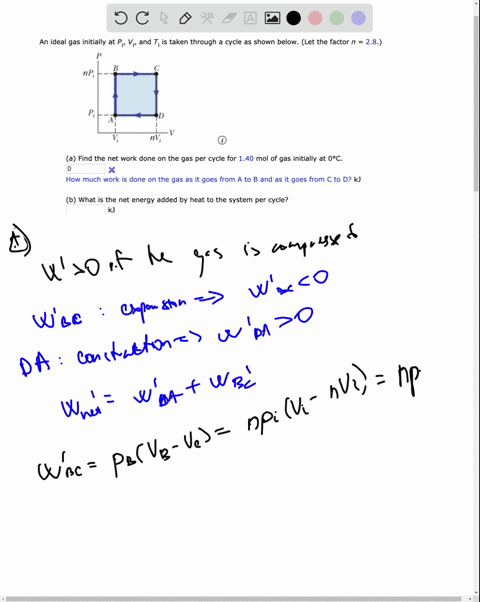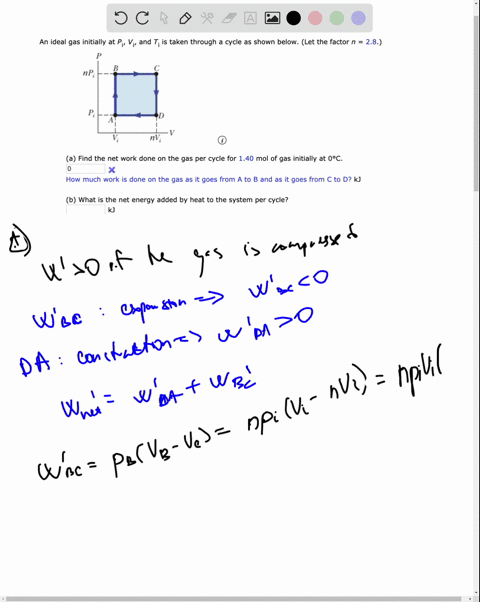
SOLVED: An ideal gas initially at Pi' Vi' and Ti is taken through a cycle as shown below. (Let the factor n 2.8.) nP; nV; a) Find the net work done on

A Quantum Theory for Bose–Einstein Condensation of the Ideal Gas – Quantum

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of

Solved An ideal gas initially at Pi, Vi, and Ti is taken
Ideal Gas Graph Sketching
Two moles of an ideal gas is compressed isothermally and reversibly from a volume 2L to 0.5L at initial pressure of 1 atm . the work done by gas i
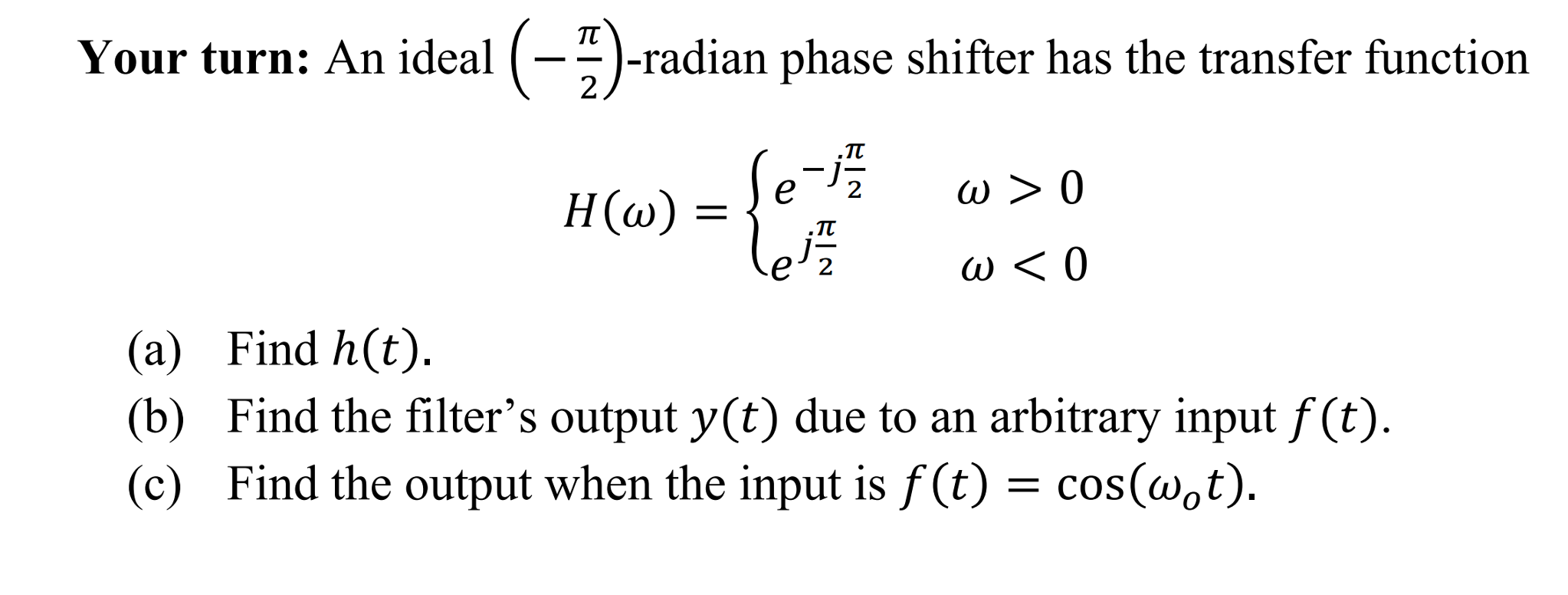
- Solved An ideal (-pi/2)-radian phase shifter has the
- Sorority Shop Alpha Omicron Pi Jewelry Dish - Heart-Shaped High-gloss finish Ceramic tray with Gold Detailing, Multi-Function Ceramic Ring Dish for Home or Office, Ideal for Jewelry and Keys : Clothing

- Apple Pi Shirt for Pi Day - Math Teacher Gift Idea

- Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure

- Бюстгальтер ортопедический PI-iV

- Levi's CEO Washes Jeans in Shower, Not Washing Machine
- Women's High-Rise Slim Fit Effortless Pintuck Ankle Pants - A New Day™ Green 2
- Nike Womens Purple Pro Indy Dry Sports Bra Size Small L52348

- Second Best Presents New PUMA Clyde Collaboration

- Women's X-Lady Premium Heart Print Dil Bra For Women And Girls

