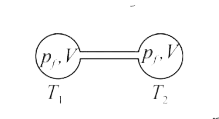
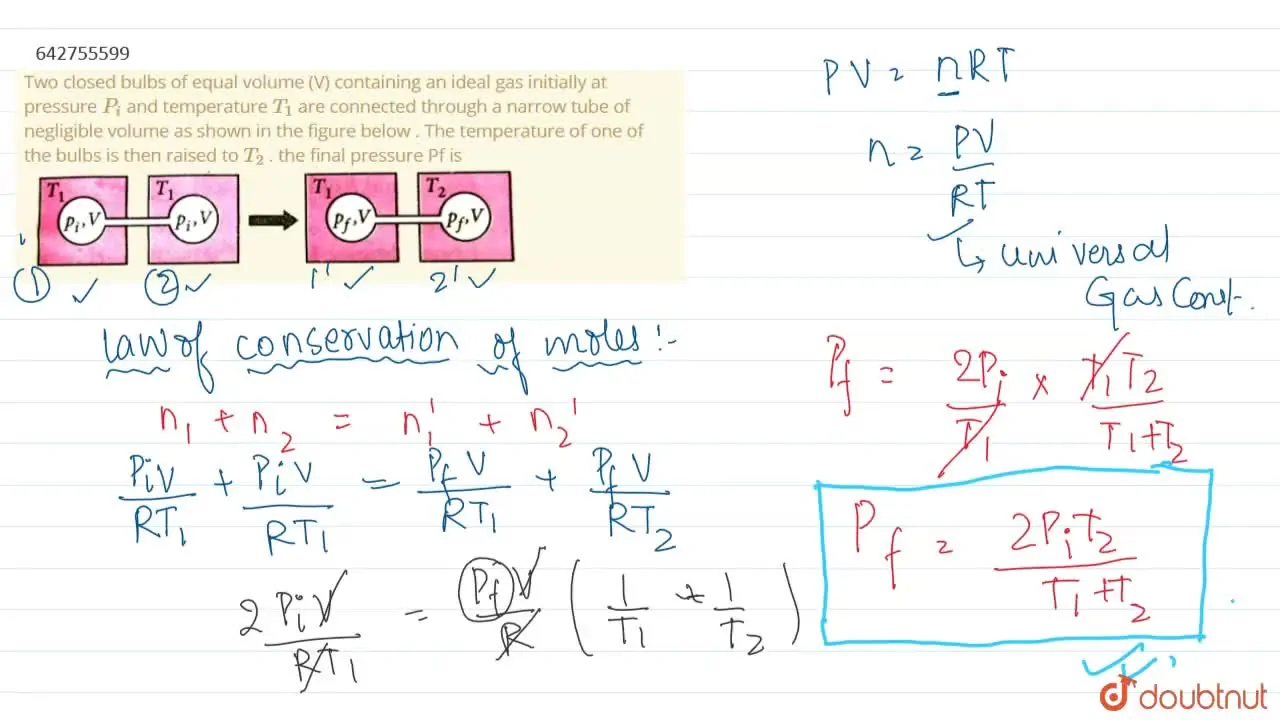
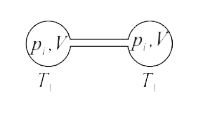
Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure

By A Mystery Man Writer
Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T2. The final pressure pf is :
Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below- The temperature of one of the bulbs is then raised to T2- The final pressure pf is -
Since the above system is a closed one, the total number of moles of the ideal gas will be equal before and after the temperature increase.
Hence in the given c

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
Two closed bulbs of equal volume (V) containing an ideal gas initially
NEET Practice Test - 22 Free MCQ Practice Test with Solutions - NEET

Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure

IIT-JEE Mains 2016 Offline Previous Question Paper Set E

Air is blown through a hole on a closed pipe containing liquid. Then the pressure will [AFMC 2005]
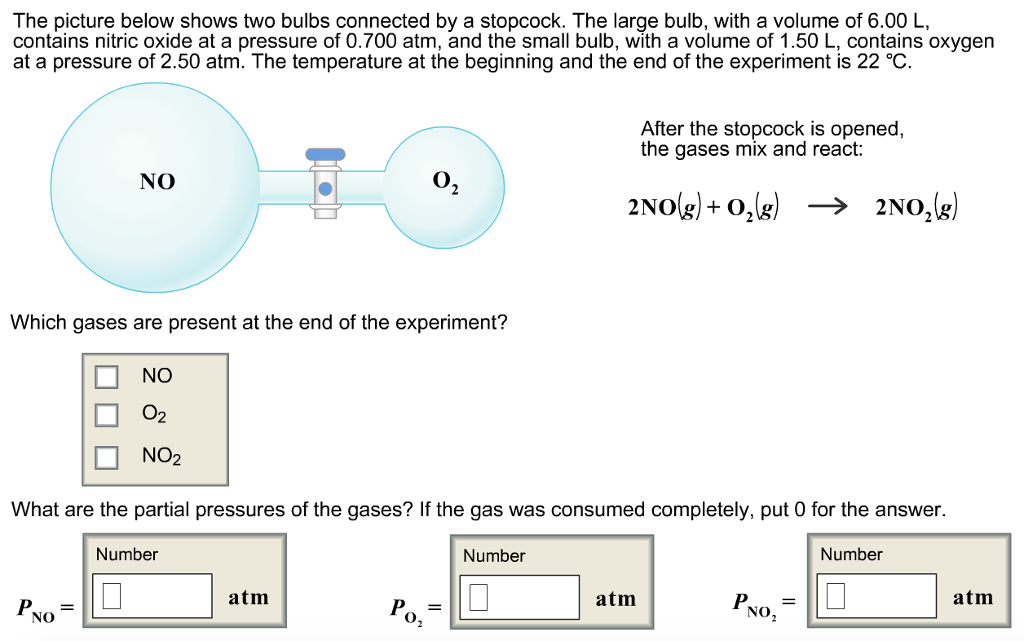
Solved The picture below shows two bulbs connected by a
The volume-temperature graphs of a given mass of an ideal gas at const
Two closed bulbs of equal volume (V) containing an ideal gas initially
Two closed bulbs of equal volume (V) containing an ideal gas initially
- PI Ideal Post-Operative Compression Bra

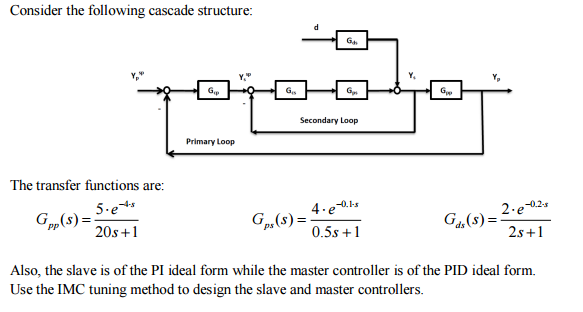
- Consider the following cascade structure: The
- Pi day idea for a poster or postcard Royalty Free Vector

- 15.6inch Slim Laptop Based on Raspberry Pi Compute Module, Ideal For Programming Learning (WS-18283)

- EDIMAX - Legacy Products - Wireless Adapters - N150 Wi-Fi Nano USB Adapter, Ideal for Raspberry Pi

- Anita 36E Twin Firm Maximum Comfort Wire-Free Bra 5695 SKIN IN BOX

- 44D Size Bra - Buy 44D Orange Bra Online

- Buy SOFT SILHOUETTES NON PADDED NON WIRED MAROON BRA for Women
- X-Fit Legging Final Sale - Five The Label

- KO Sports Gear Grappling Socks for Jiu Jitsu, MMA, Karate and any mat sport (Multi-color, XX-Small : Shoe Size 0 to 1) : Sports & Outdoors
