Write the expression for the compressibility factor (Z) for one

By A Mystery Man Writer
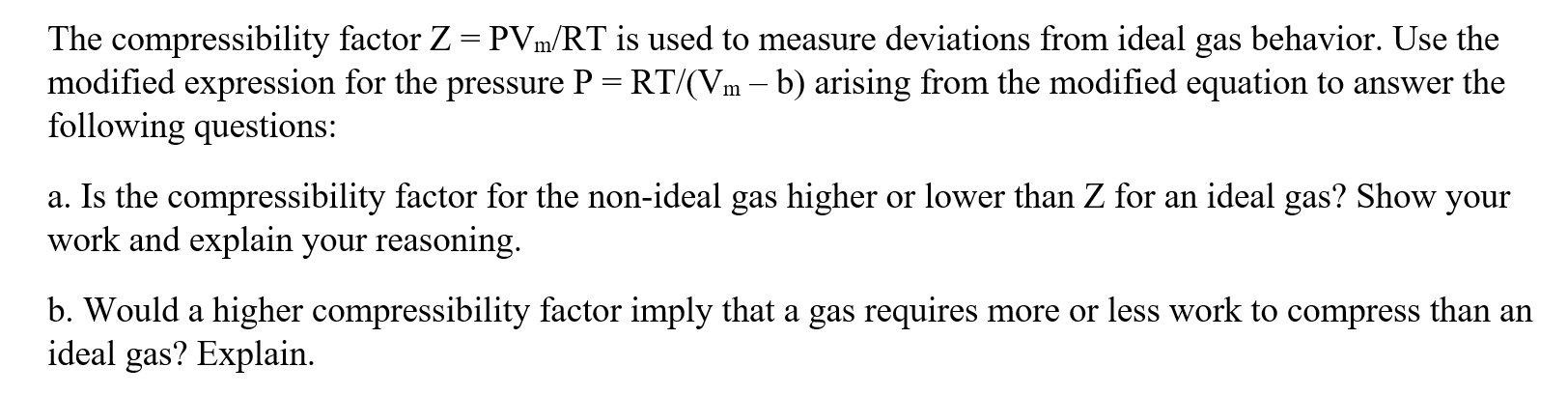
Solved The compressibility factor Z = PVm/RT is used to

1st Pu Mid Term Question Paper

1st PUC Chemistry Model Question Paper 5 with Answers (Old Pattern) - KSEEB Solutions
Chapter 5 states_of_matter (1)-converted_2639.pdf - Chemistry - Notes - Teachmint

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))

SOLVED: Problem 2. This problem involves the derivation of the

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

5.2 Mass and energy conservation equations in a control volume – Introduction to Engineering Thermodynamics

Solved The van der Waals equation of state can be used to

If Z is a compressibility factor, van der Waals equation at low pressure ..
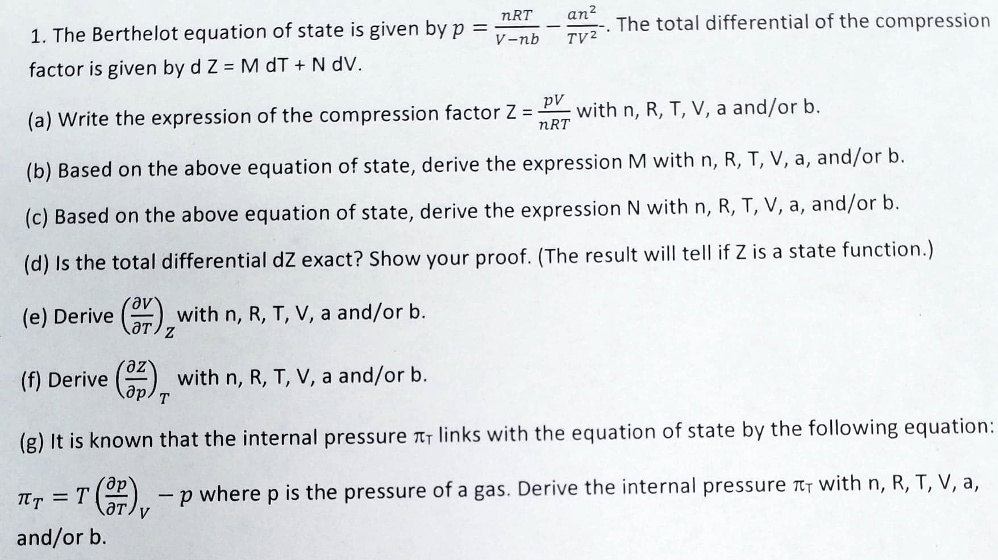
SOLVED: The Berthelot equation of state is given by: p = nRT / (V - nb) The total differential of the compression factor Z is given by: dZ = MdT + NdV (

1st PUC Chemistry Model Question Paper 5 with Answers (Old Pattern) - KSEEB Solutions

Kannada] Explain the significance of compressibility factor.
- Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

- Compressibility factor (z): real gases deviate from ideal behav-Turito

- Chemistry Desk: Effect of Pressure

- Compressibility factor Z as function of temperature T with lines of

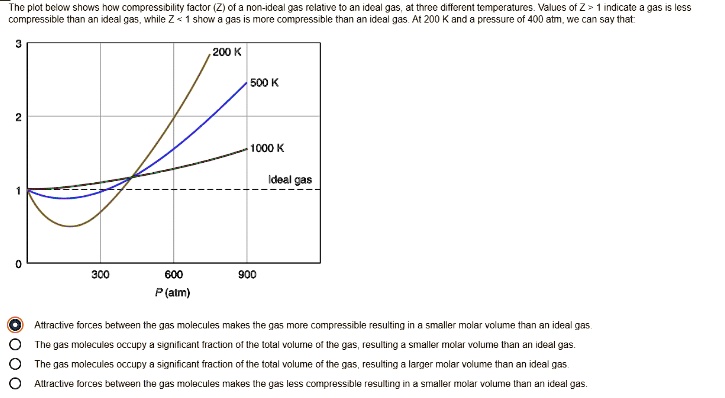
- SOLVED: Plot bclon shcs now compressibility factor (Ziofa non-Idc? 935 relative to an ideal gas; J1 force differential Mocraiurc: Values of Z indicate compressibility and inan re any more compressible ideal gas
- Vanity Fair Beauty Back Underarm & Back Smoother T-Shirt Bra & Reviews

- Flowy Open Sides Athletic Skort - Magenta – Moda Boutique

- Undies for 2 - Fundies Underwear Bachelor Party - QTY DISCOUNTS - FREE GIFT

- Como Usar o Gift Card Spotify Premium

- Wrangler Mens Cowboy Cut Jean Original Fit - Stonewashed – Broken horn
