physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

By A Mystery Man Writer
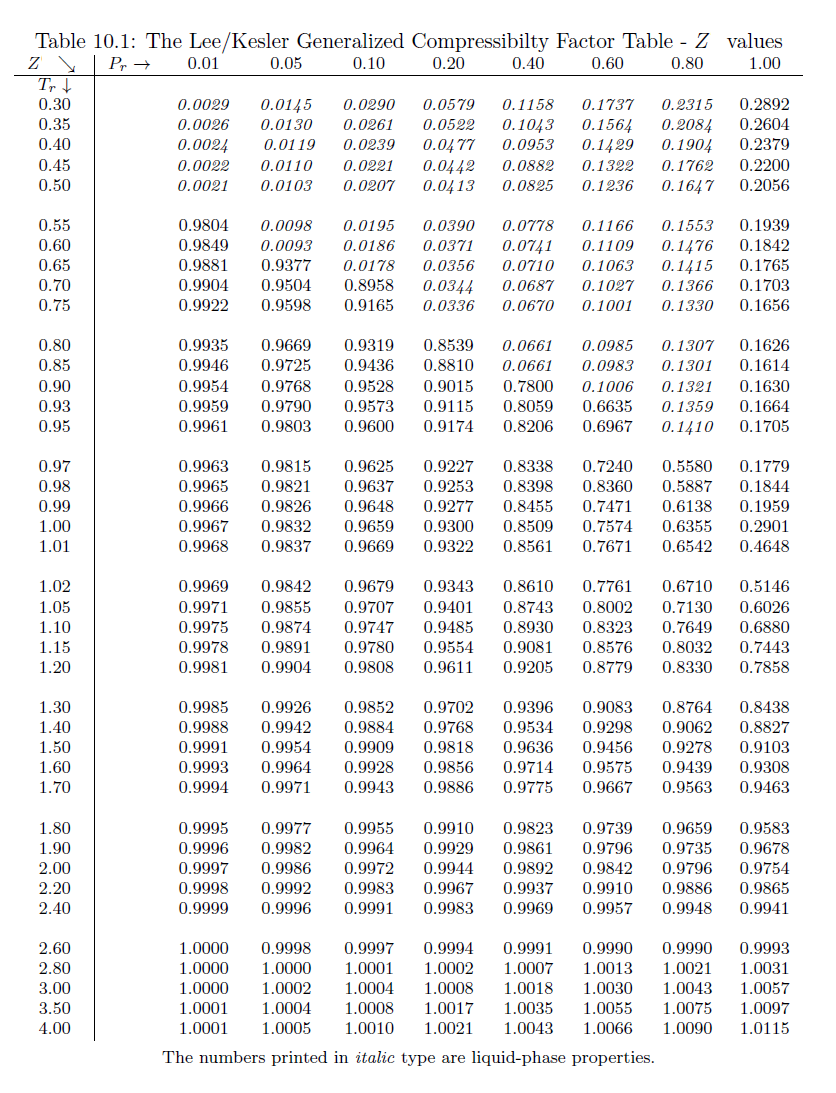
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In
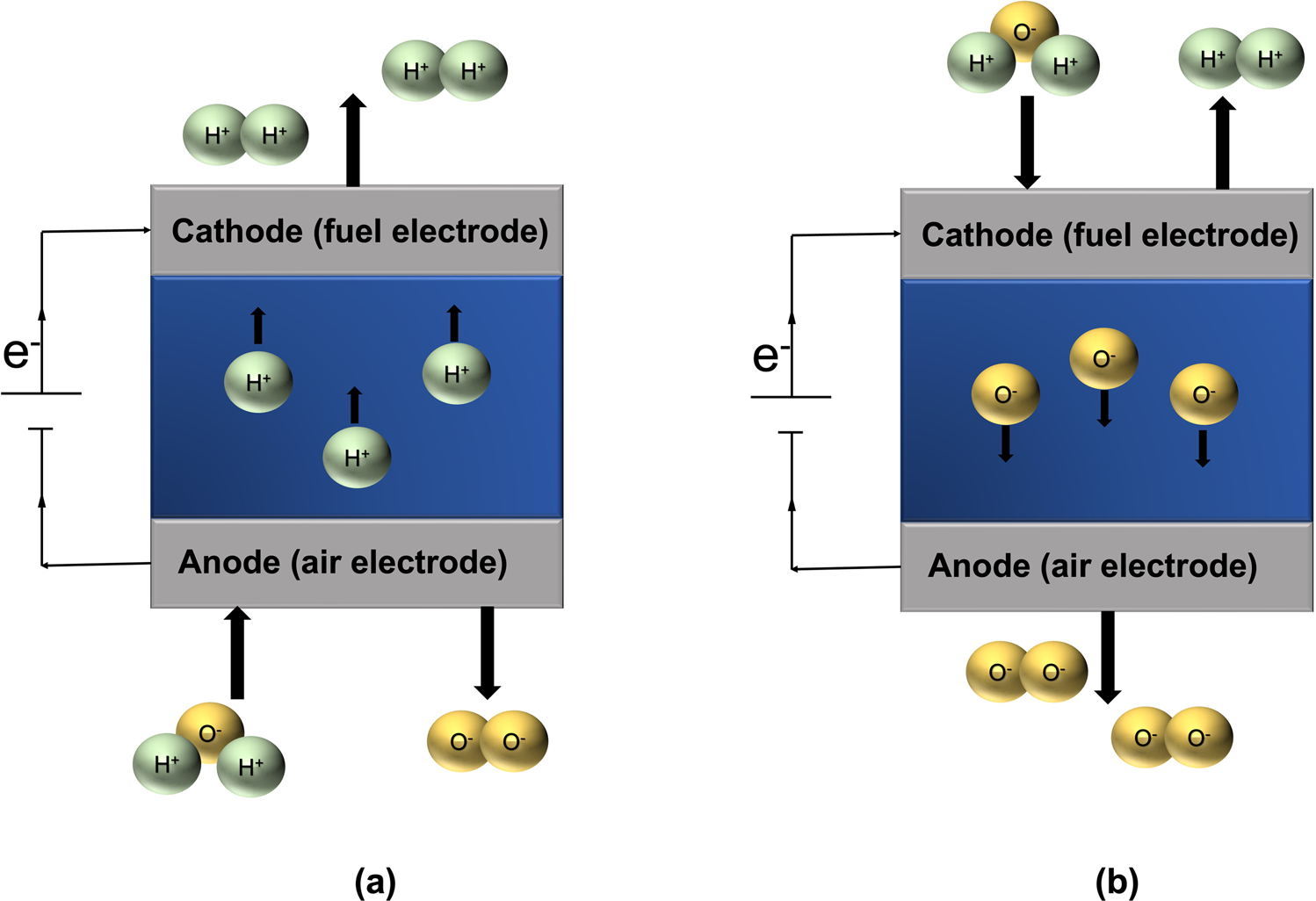
Enhancing the Faradaic efficiency of solid oxide electrolysis

physical chemistry - Why is the excluded volume in van der Waals 4

physical chemistry - why is the pressure exerted by ideal gas on

3.2 Real gas and compressibility factor – Introduction to

Preliminary Chemical Engineering Plant Design - William - Ventech!

VISIONS OF A HYDROGENECONOMY - THE ELEMENT HYDROGEN

Ideal gas - Wikipedia

The compressibility factor Z a low-pressure range of all gases
Energies, Free Full-Text
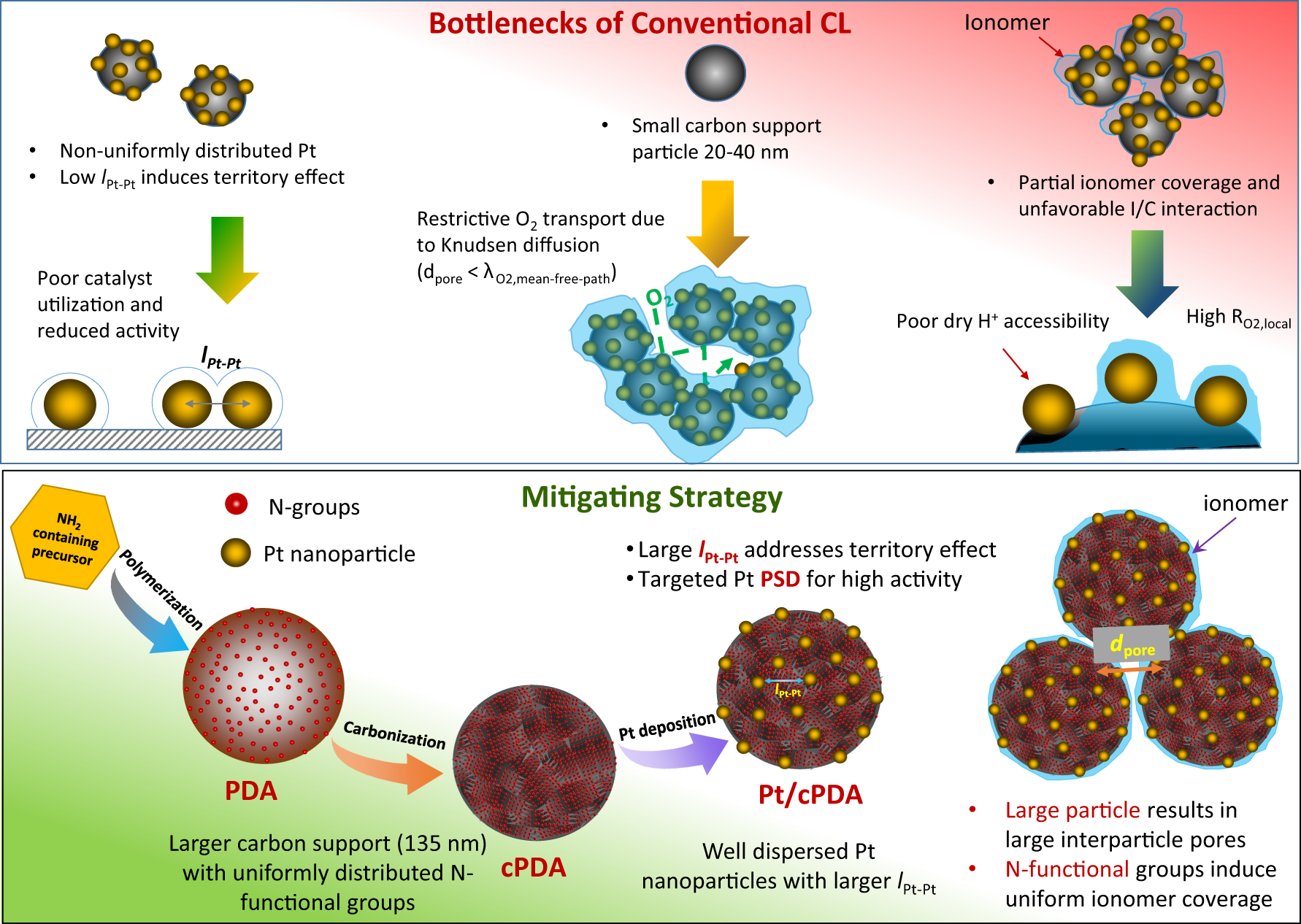
Designing fuel cell catalyst support for superior catalytic
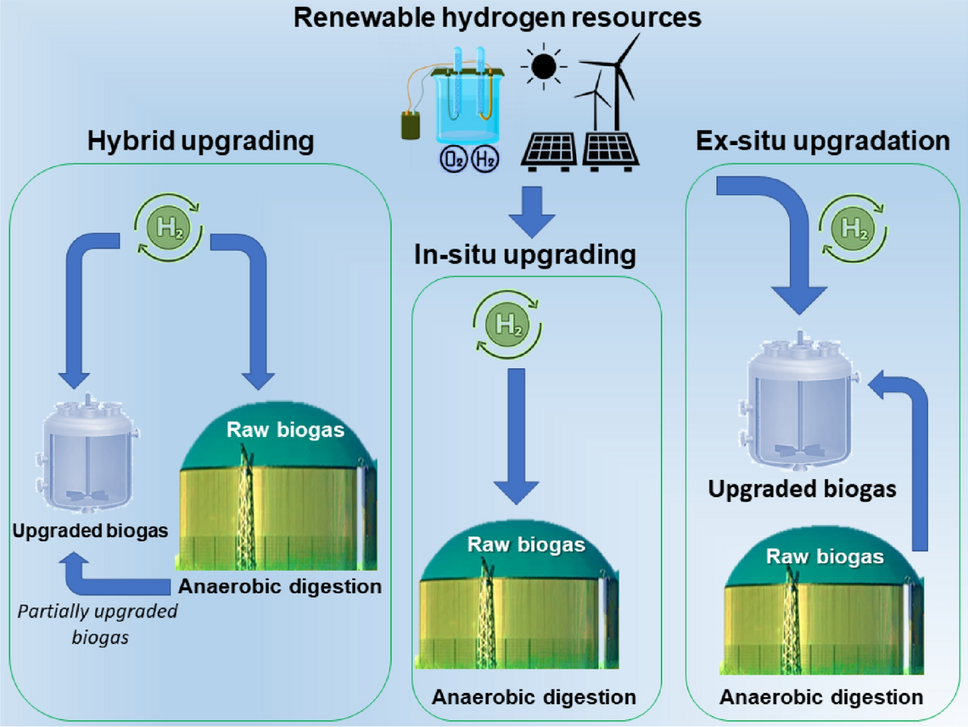
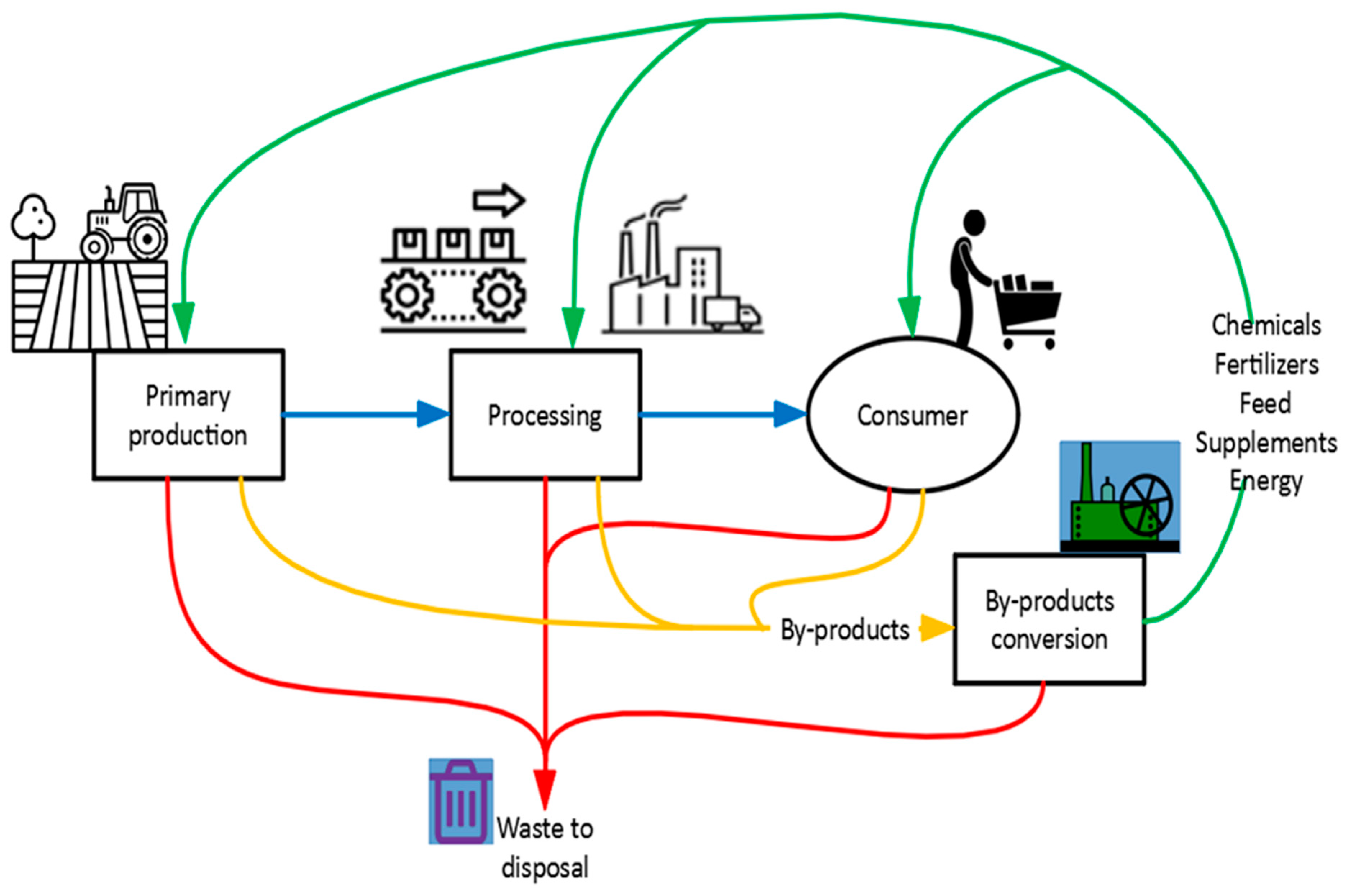
Integration of biogas systems into a carbon zero and hydrogen

Heat pump - Wikipedia