Protocol for a randomised controlled feasibility trial of exercise
By A Mystery Man Writer
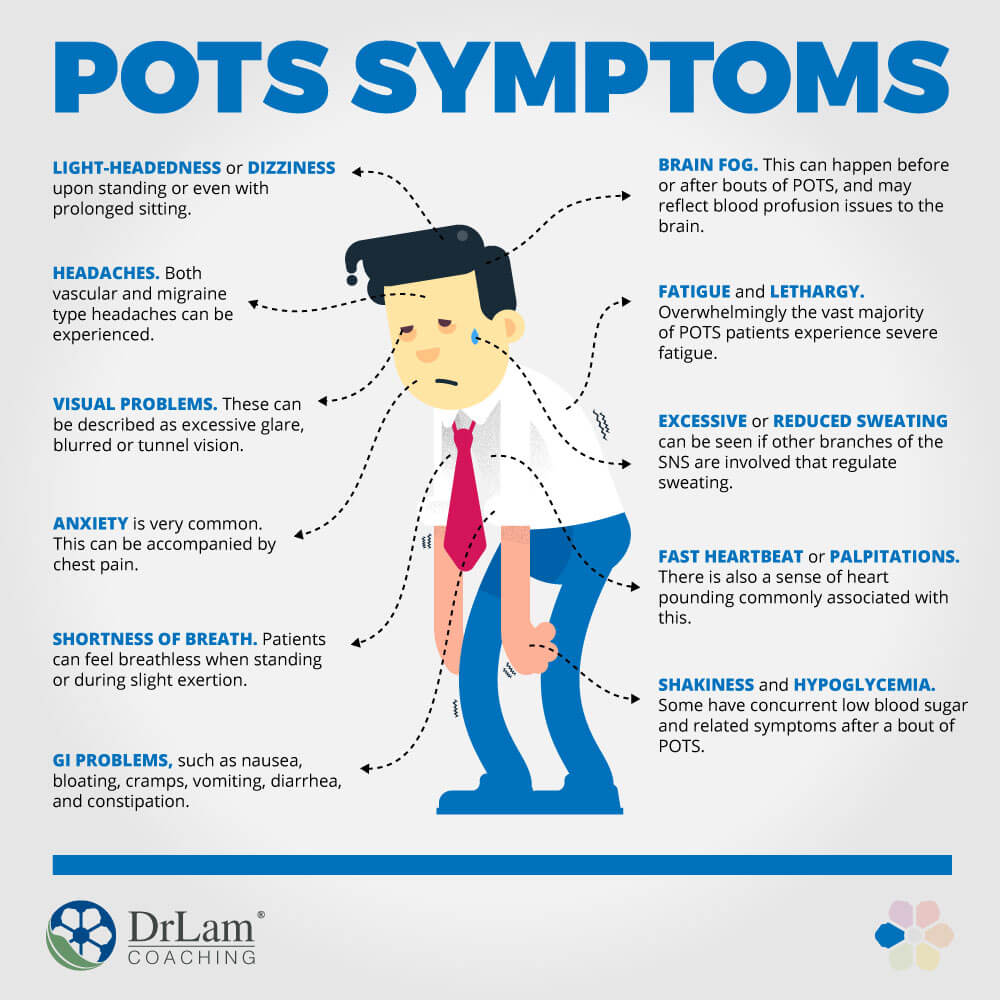
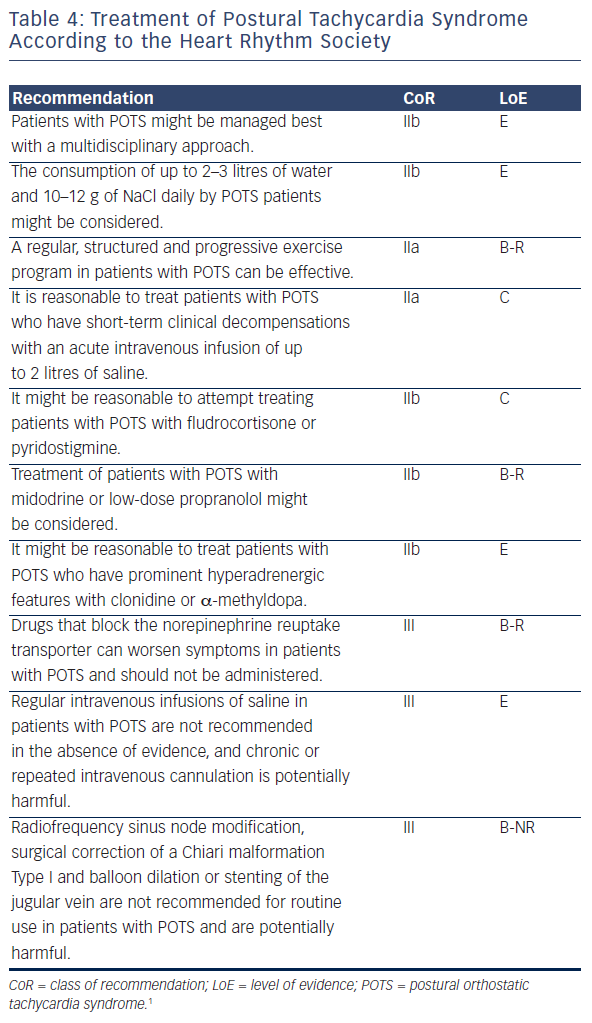
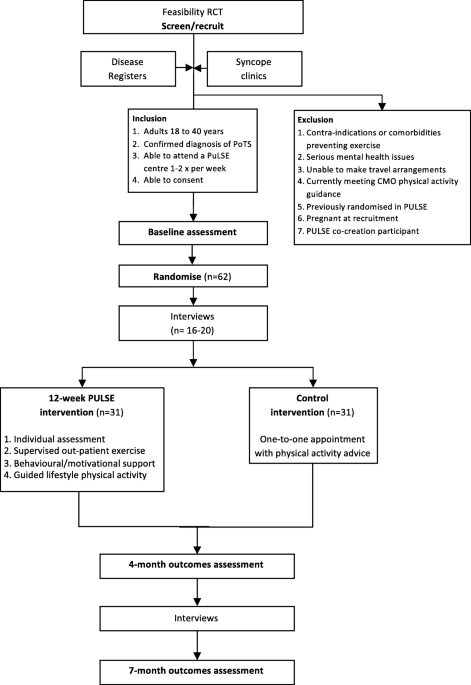
Background Postural orthostatic tachycardia syndrome (POTS) is an autonomic nervous system disorder causing an abnormal cardiovascular response to upright posture. It affects around 0.2% of the population, most commonly women aged 13 to 50 years. POTS can be debilitating; prolonged episodes of pre-syncope and fatigue can severely affect activities of daily living and health-related quality of life (HRQoL). Medical treatment is limited and not supported by randomised controlled trial (RCT) evidence. Lifestyle interventions are first-line treatment, including increased fluid and salt intake, compression tights and isometric counter-pressure manoeuvres to prevent fainting. Observational studies and small RCTs suggest exercise training may improve symptoms and HRQoL in POTS, but evidence quality is low. Methods Sixty-two people (aged 18–40 years) with a confirmed diagnosis of POTS will be invited to enrol on a feasibility RCT with embedded qualitative study. The primary outcome will be feasibility; process-related measures will include the number of people eligible, recruited, randomised and withdrawn, along with indicators of exercise programme adherence and acceptability. Secondary physiological, clinical and health-related outcomes including sub-maximal recumbent bike exercise test, active stand test and HRQoL will be measured at 4 and 7 months post-randomisation by researchers blinded to treatment allocation. The PostUraL tachycardia Syndrome Exercise (PULSE) intervention consists of (1) individual assessment; (2) 12-week, once to twice-weekly, supervised out-patient exercise training; (3) behavioural and motivational support; and (4) guided lifestyle physical activity. The control intervention will be best-practice usual care with a single 30-min, one-to-one practitioner appointment, and general advice on safe and effective physical activity. For the embedded qualitative study, participants (n = 10 intervention, n = 10 control) will be interviewed at baseline and 4 months post-randomisation to assess acceptability and the feasibility of progressing to a definitive trial. Discussion There is very little high-quality research investigating exercise rehabilitation for people with POTS. The PULSE study will be the first randomised trial to assess the feasibility of conducting a definitive multicentre RCT testing supervised exercise rehabilitation with behavioural and motivational support, compared to best-practice usual care, for people with POTS. Trial registration ISRCTN45323485 registered on 7 April 2020.

PDF] Endurance Exercise Training in Orthostatic Intolerance: A Randomized, Controlled Trial

Study protocol for a randomised controlled trial evaluating the effectiveness of strengths model case management (SMCM) with Chinese mental health service users in Hong Kong

Feasibility & Study Start-Up at SCOPE Summit

Nikki Holliday - Design Manager - Coventry University

PDF) Combined online interactive mindfulness and exercise programme (MOVE-Online) compared with a self-management guide for adults with chronic pain: protocol for a randomised controlled feasibility trial

First Randomized Controlled Trial Comparing Woebot to Clinician-Led Psychotherapy Reveals Digital Mental Health Intervention Is Non-Inferior in Reducing Depressive Symptoms Among Teens

Protocol for a randomised controlled feasibility trial of exercise rehabilitation for people with postural tachycardia syndrome: the PULSE study, Pilot and Feasibility Studies

157757 PDFs Review articles in HEALTH-RELATED QUALITY OF LIFE

A study protocol for a randomised controlled feasibility trial of an intervention to increase activity and reduce sedentary behaviour in people with severe mental illness: Walking fOR Health (WORtH) Study

mLearning in the Democratic Republic of the Congo: A Mixed-Methods Feasibility and Pilot Cluster Randomized Trial Using the Safe Delivery App

Semi-supervised exercise training program more effective for individuals with postural orthostatic tachycardia syndrome in randomized controlled trial

Exercise Guidelines for Postural Tachycardia Syndrome

PDF] Study within a trial (SWAT) protocol. Investigating the effect of personalised versus non-personalised study invitations on recruitment: An embedded randomised controlled recruitment trial

Clinical Trial Designs & Clinical Trial Phases

Nikki Holliday - Design Manager - Coventry University
- Adrienne Vittadini Riding High Faux Croco 4-Piece Luggage Set

- 500 push-ups a Day: The Ultimate Challenge for Building Upper Body Strength
- OEM Adult Pull up Diapers Overnight Pull UPS Pull up Diapers Best

- H&M Divided Jogger Pants Men's Large Blue Comfort Waist Stretch Drawstring

- My Favorite Bra I've Bought Over and Over for Over a Decade