gas laws - How to find the temperature relationship between the

By A Mystery Man Writer
The following graph denotes the variation of the compressibility factor (Z) with pressure at different temperatures for a real gas. Simply each of the curves represents an isotherm. Now, suppose w

1. PV = n RT (Ideal gas law)2. You are given that 273.15 K is the .pdf
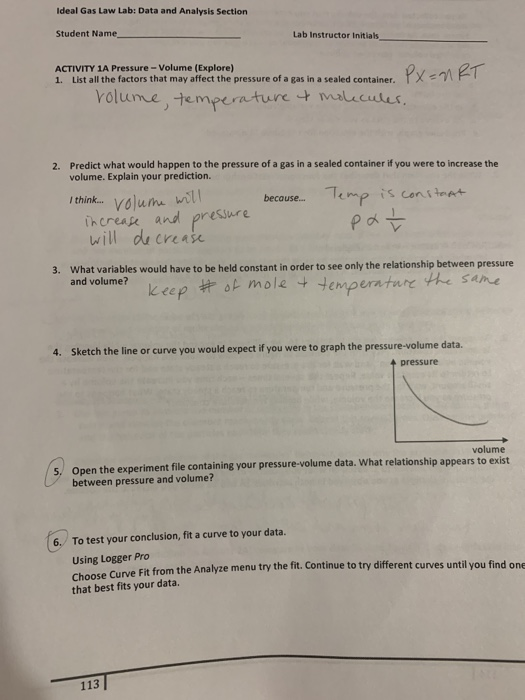
Solved Ideal Gas Law Lab: Data and Analysis Section Student

Gas Laws
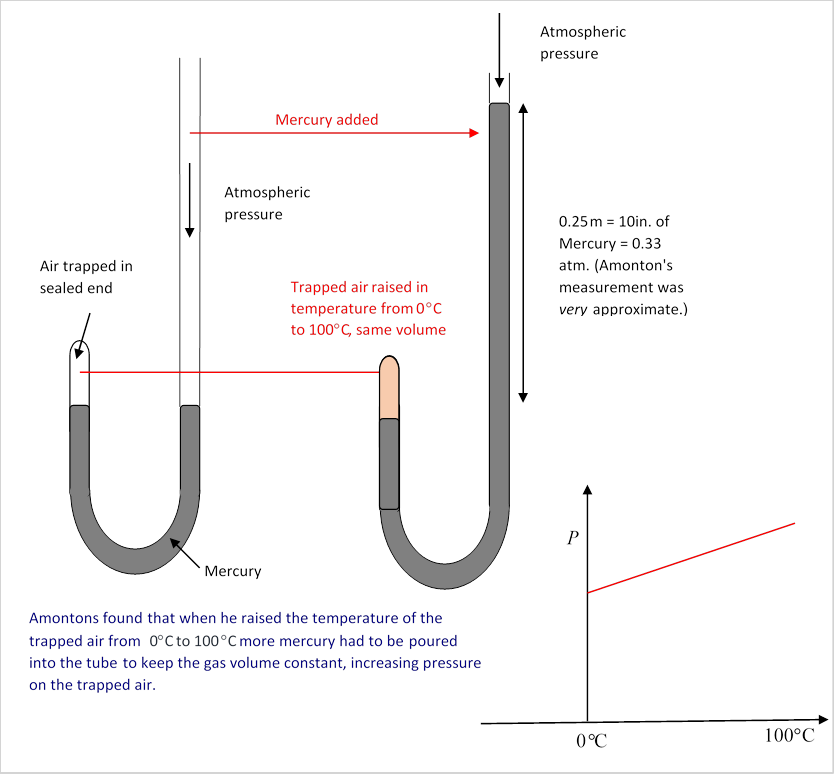
Gas Law and Avogadro
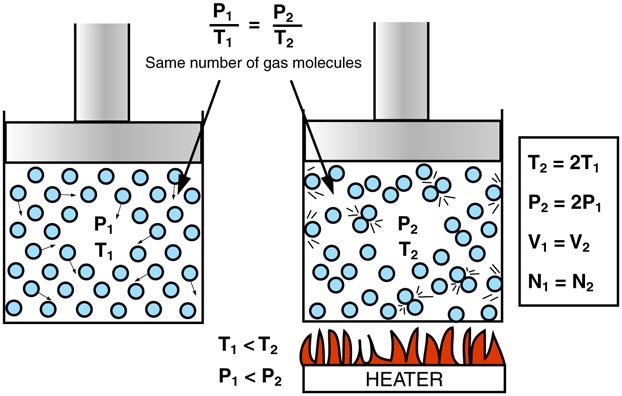
Gas Laws

Ideal Gas Law Calculator PV = nRT
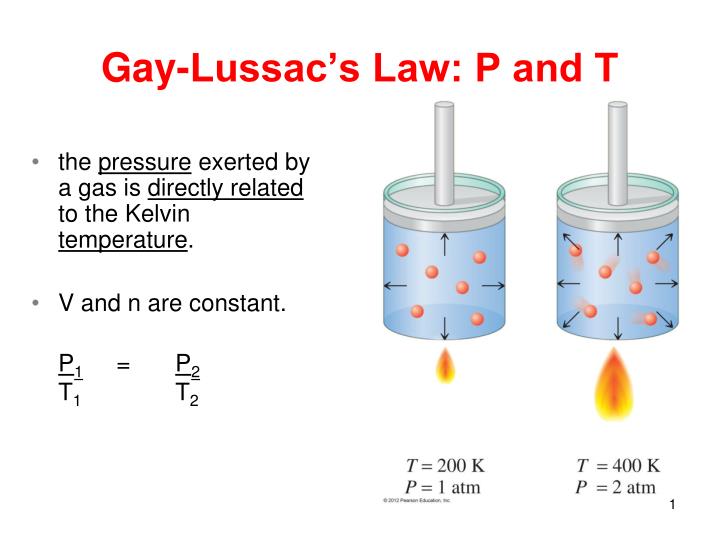
If you want to study the relationship between temperature and pressure of a gas, which factor must be held constant?

8.2 Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law – Chemistry

Ideal Gas Law Formula and Examples

PV=nRT - Use the Ideal Gas Law Ideal gas law, Gas constant, Chemistry 101

Ideal gas law, Definition, Formula, & Facts

Ideal Gas Law: relationship between pressure, temperature, volume, number of molecules; when given volume, temp,…
- Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

- The role of the compressibility factor Z in describing the
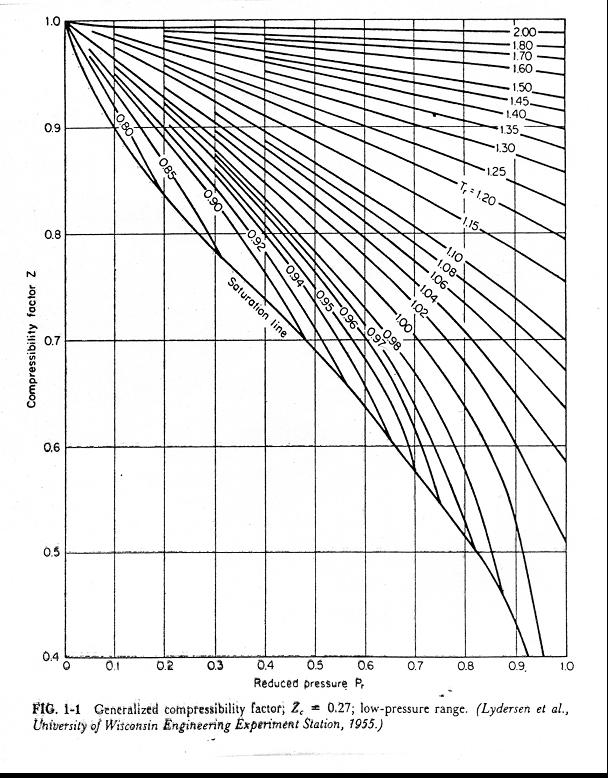
- Objectives_template

- The compressibility factor Z a low-pressure range of all gases

- In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

- Letras de Decoração de Madeira. Letras Scrabble Letras Tipo Scrabble em madeira.

- Bite Correction Dental Beauty - Premier Dental Office in
- JubileeYarn Undyed Yarn - 45% Bamboo 40% Wool 15% Nylon -100g/460yds - 3 Pack

- If Maidenform happens to be your bra of choice, this blogger

- Flawless Comfort Wire Free Crop
