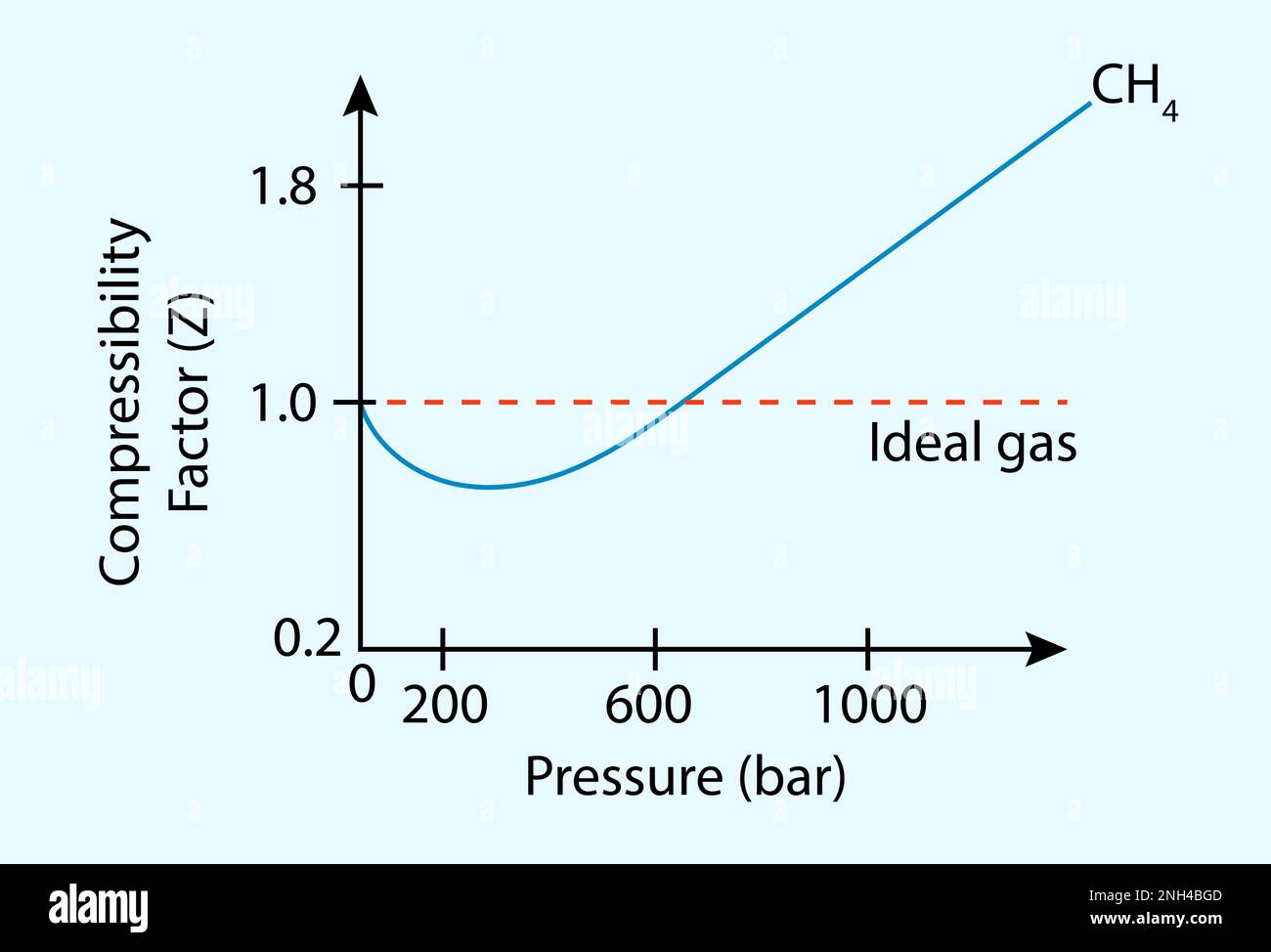
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

By A Mystery Man Writer
In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

P k nag solution by Shaikh Mohd Aslam - Issuu

The graph of compressibility factor (Z) vs. P for one mole of a
Compressibility hi-res stock photography and images - Alamy

KVPY-SX 2016 Chemistry Question Paper with Solutions PDF Download

In following compressibility factor versus pressure graph which is
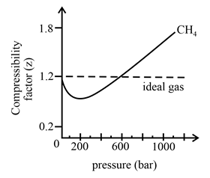
In the following compressibility factor Zvs pressure graph at 300Kthe compressibility of CH4 at pressures 200bardeviates from ideal behaviour because

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
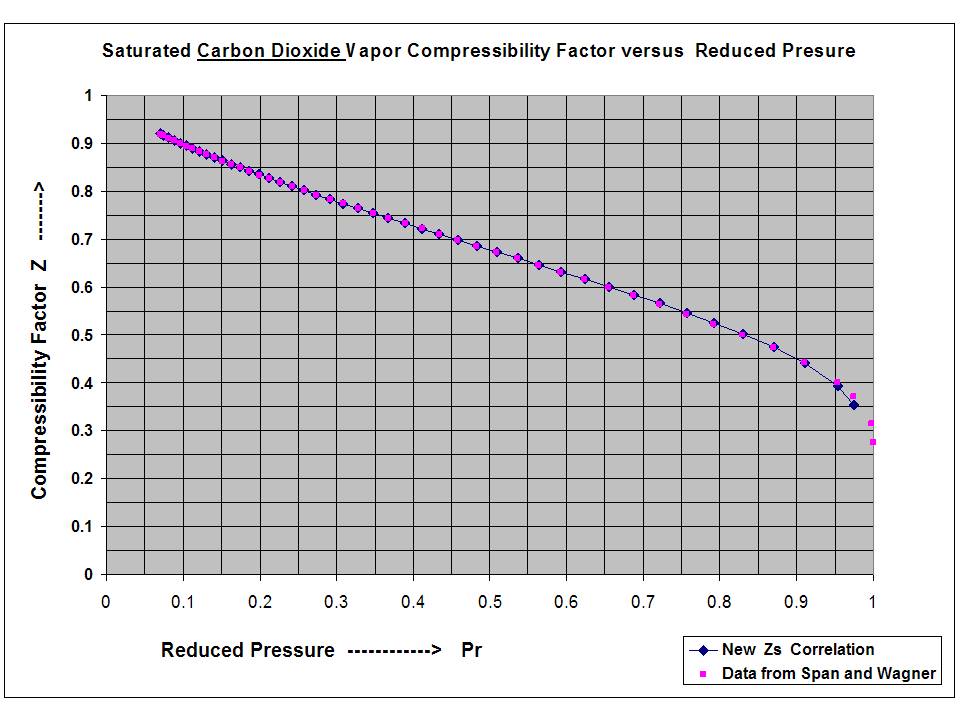
New compact Equations for the Compressibility Factor Z and Density of Liquid and Vapor Carbon Dioxide

In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
- Math cad compressibility factor, z, of real gas using the redlich-kwong equation of state

- Gas Compressibility - an overview

- Compressibility factor (Z) is plotted against pressure at different te

- Compressibility factor Z as function of temperature T with lines of

- What to Wear with White Lululemon Shorts: Style Guide - Playbite

- J. Jill, Tops

- DEERE 50D Mini (up to 12,000 lbs) Excavators For Sale
- Women Honeycomb Anti Cellulite Leggings High Waist Yoga Pants Bubble Textured Scrunch Ruched Butt Lift Running Tights Plus Size

- Women Waterproof Ski Pants Faux Pants Leggings Stretch Waisted Women's High Leather Pleather Pants Business Casual Pants For Woman

