The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor compressibility factor for onemole of a van der waals gas at 0c
Click here👆to get an answer to your question ✍️ The compression factor -compressibility factor- one mole of a van der Waals gas 0-C and 100 atm pressure is found to be 0-5- Assuming that the volume of a gas molecule is negligible- calculate the van der Waals- constant a

Solved (Triple-Play Bonus) For a certain gas, the

6.3: Van der Waals and Other Gases - Physics LibreTexts

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is

The compression factor (compressibility factor) for one mole of a v

Sheet - 01 - Real Gas, PDF, Gases

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

79. Al high pressure, the compressibility factor one male of van

1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts


The compression factor (compressibility factor) for one mole of a Van der..
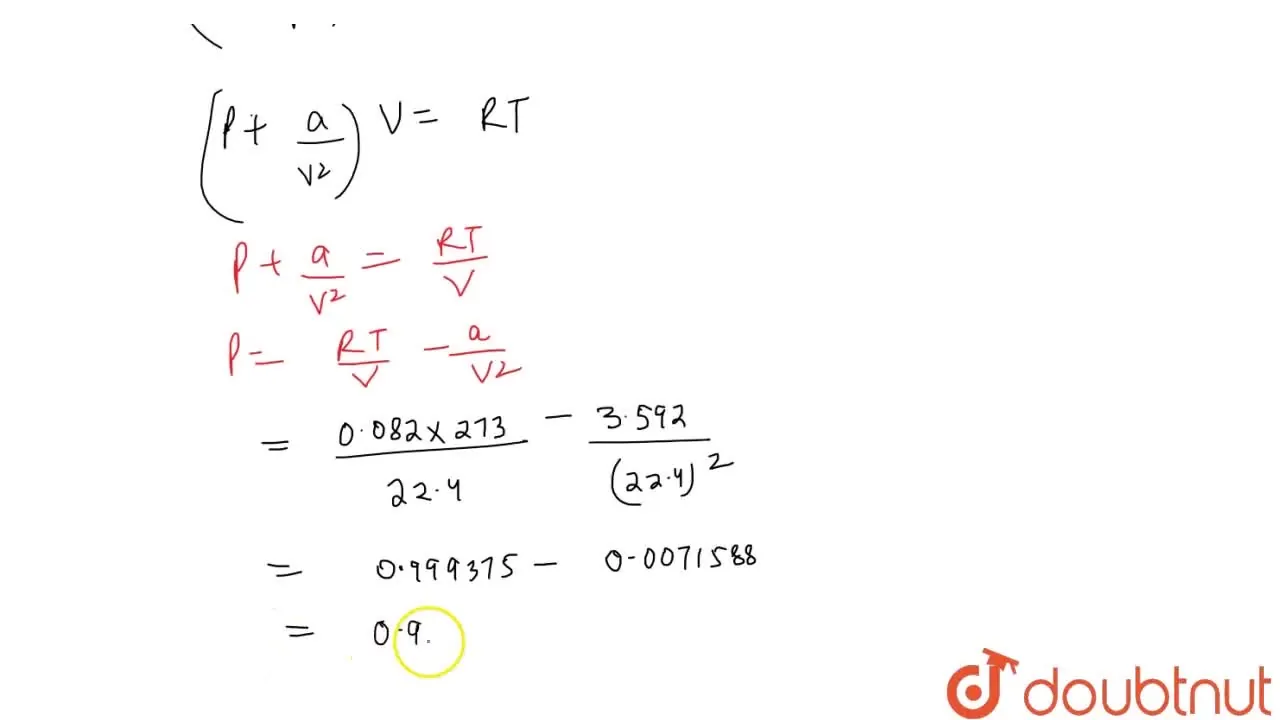
Calculate the pressure exerted by one mole of CO(2) gas at 273 K van d

The compressibility factor for definite amount of van der Waals' gas at `0 ^(@)C` and
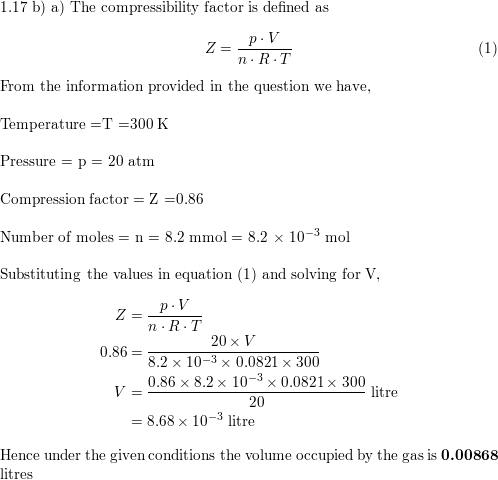
- Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

- Compressibility Factor from Redlick-Kwong Equations

- Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be

- Excel Calculations: Compressibility Factor for Natural Gas
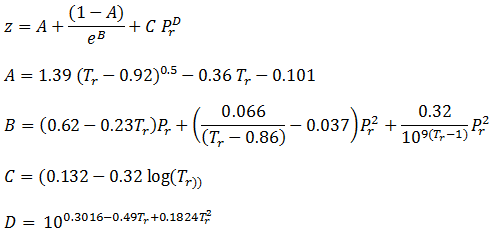
- Solved] Why is the compressibility factor less than 1 at most conditions?
- Medical Z Élégance Zbra Soutien Gorge Prothèse 90C Noir

- Lace Sexy Halter Backless Bra Seamless Backless Bralette

- Esobo Women's Bootcut Yoga Pants Work Pants Crossover Split Hem Full Length Flare Leggings with Pocket

- PGA TOUR Apparel Women's Plus Size Pull-On Golf Pant

- Up To 45% Off on US 2-4 Pack Women's Padded Sp
