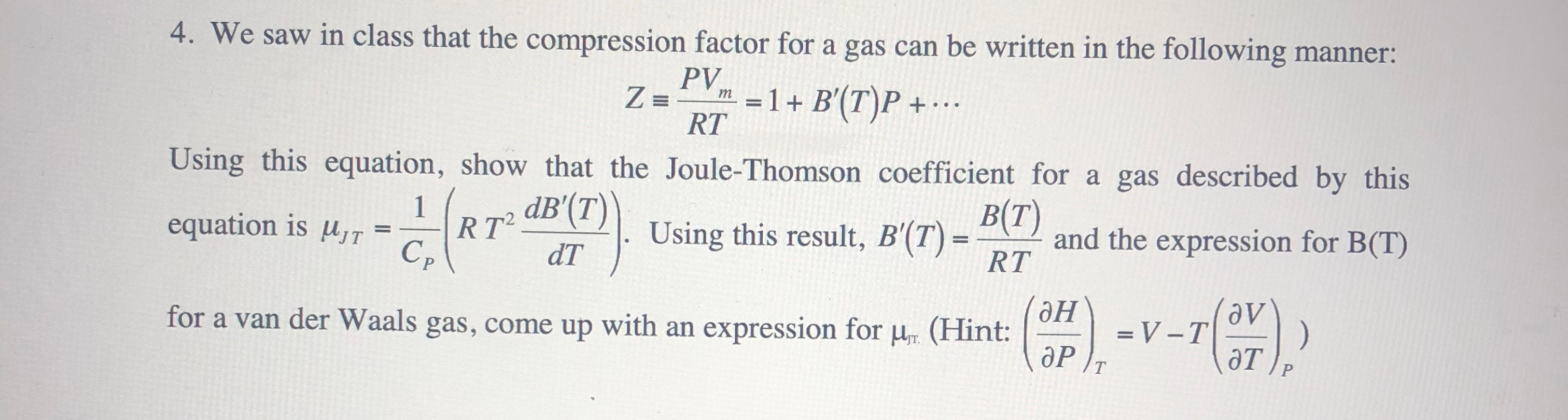
Friday, Jul 05 2024
Solved 1. The compression factor, Z of a gas is 0.625. Which
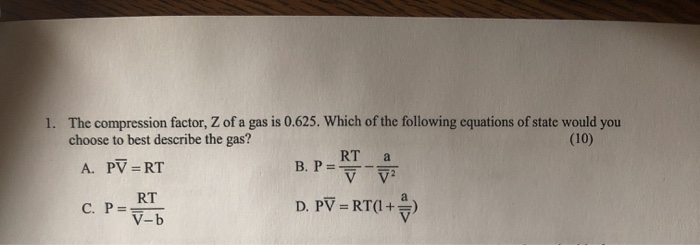
By A Mystery Man Writer

Solved 1. The compression factor, Z of a gas is 0.625. Which

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

Evolution of Permeability and Gas Seepage in Deep Coal Under 3D Stress

Solucionario faires

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1

Norriseal valve sizing reference guide by RMC Process Controls & Filtration, LLC. - Issuu

Solved NOTE: Already have answers for part a-e (a b c d

Esas 44-86, PDF, Strength Of Materials

Determine Compressibility of Gases
Related searches
Related searches
- Shop By Brand - Spa Essentials - Nail Supply Inc

- Buy Yogalicious High Waist Ultra Soft 7/8 Ankle Length Leggings

- 9 Things We Learned About Kim Kardashian from Her Assistant, Steph Shep
- Nike Sportswear Essential Fleece Cargo Big Pants Pink

- Que vitória incrível! Parabéns ao São Paulo pelo título da Supercopa do Brasil! 🎉⚽️🏆 Celebre essa conquista com a Camiseta Alignmed…
©2016-2024, doctommy.com, Inc. or its affiliates