The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement

By A Mystery Man Writer
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect
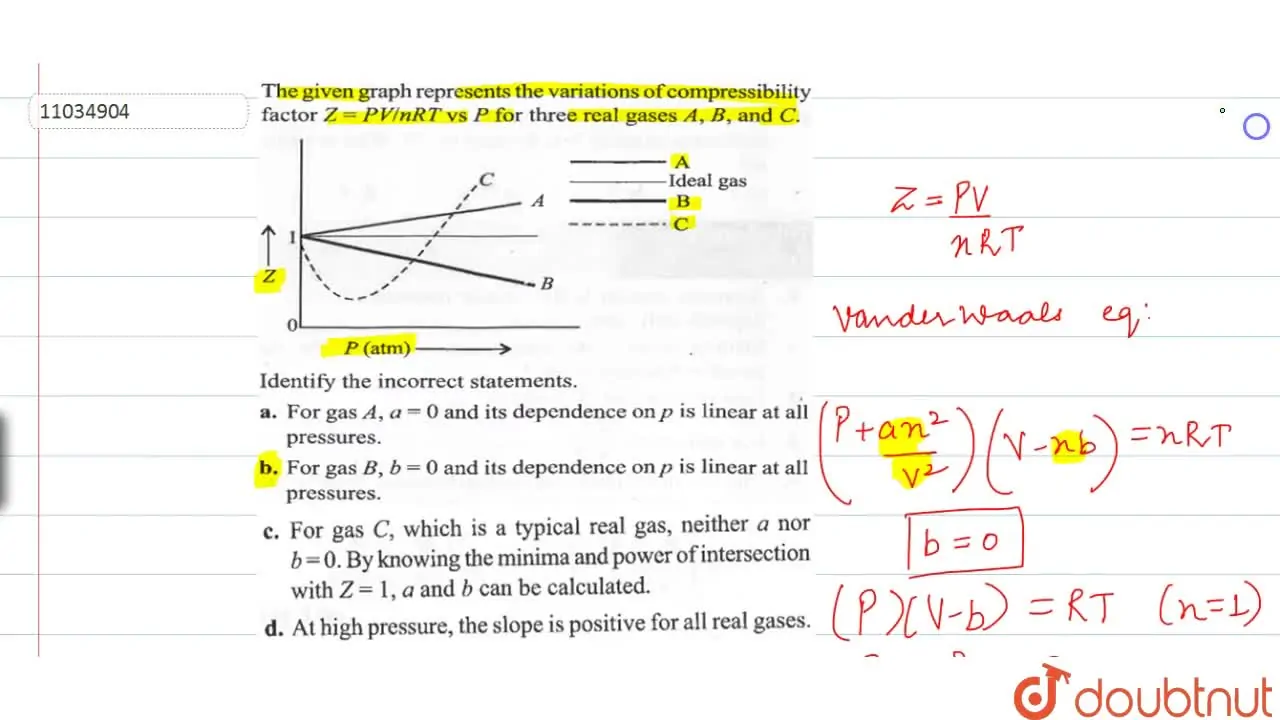
For the gas C which is typical real gas for which neither a nor b=0. B

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
.jpg?revision=1)
Gas Laws - Overview - Chemistry LibreTexts

the given graph represents the variation of Z (compressibility factor =dfrac{PV}{nRT}) versus P, three real gases A, B and C. Identify the only correct statement.For the gas A, a = 0 and
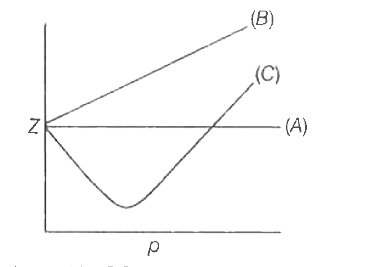
Telugu] The variation of compressibility factor (Z) with pressure (p

Compressibility factor - Wikipedia

The given graph represent the variations of Z (compressibility factor (Z)=dfrac {pV}{nRT}) versus P, three real gases A, B and C. Identify the only incorrect statement.For the gas B, b=0 and its

6.3: Van der Waals and Other Gases - Physics LibreTexts
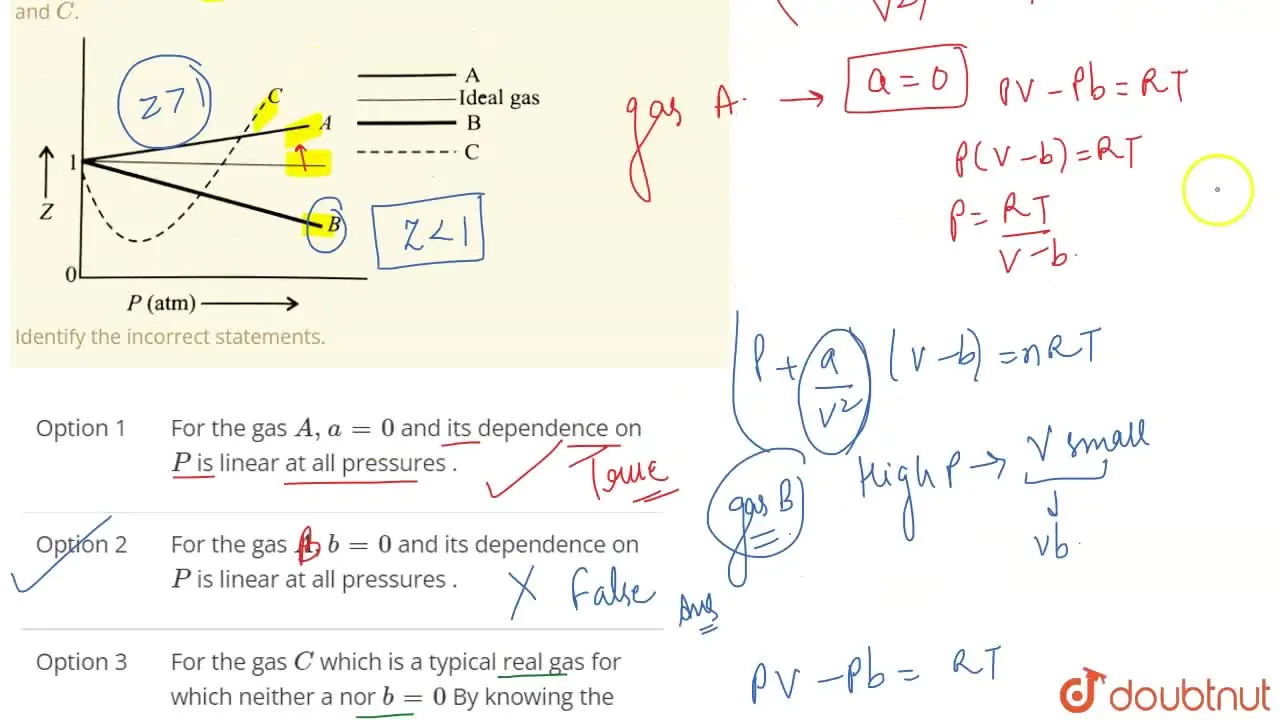
For the gas C which is a typical real gas for which neither a nor b =0


Real Gases, PDF, Gases
- Compressibility Factor Calculator

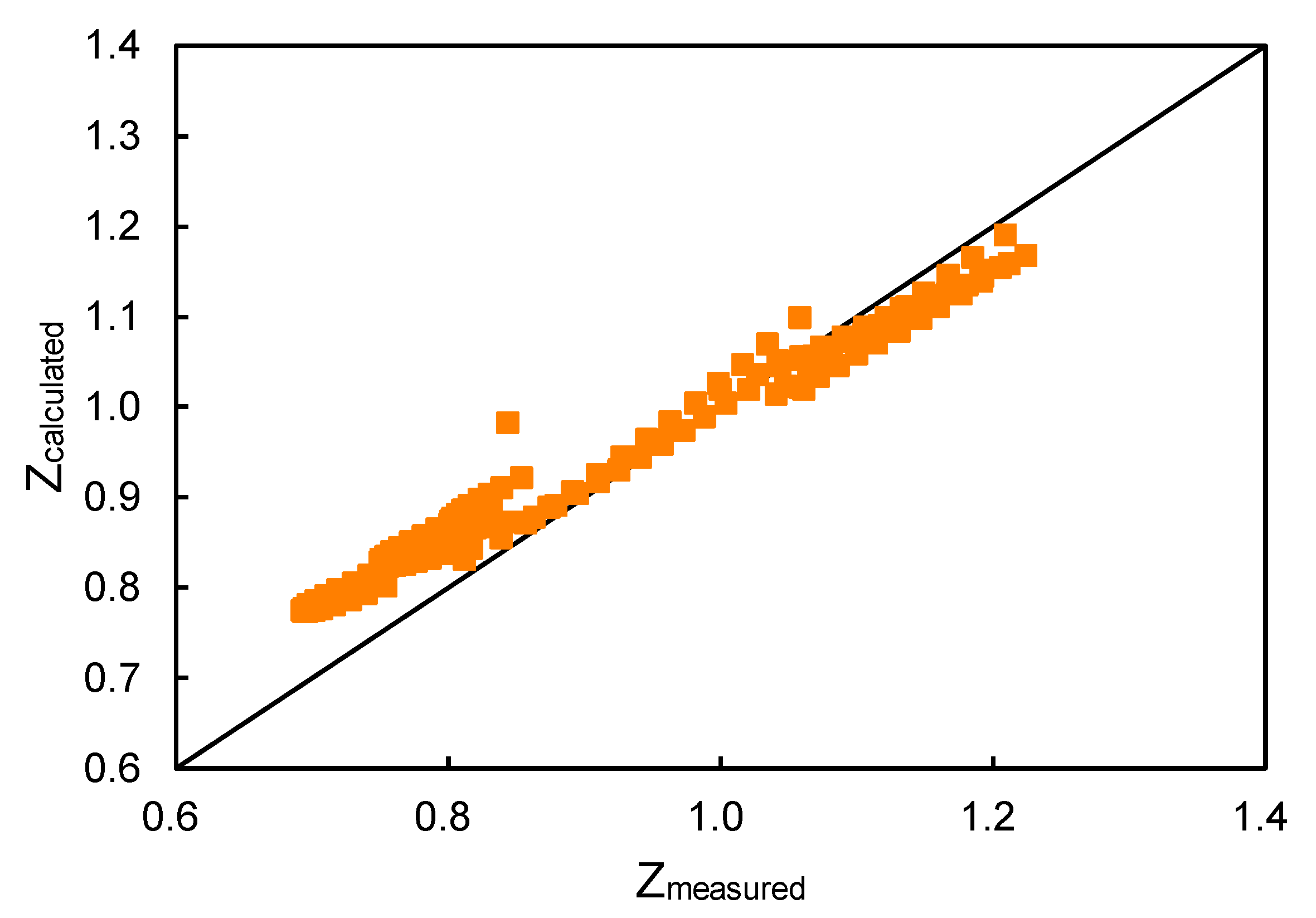
- In the following compressibility factor (Z) vs. pressure graph 300

- Energies, Free Full-Text
- Gas Z Factor Calculator: Dranchuk-Abou-Kassem · PVT Solver

- 1. What is meant by compressibility factor, Z? 2. What is the significance of compressibility factor? - Sarthaks eConnect
- JT ProFlex Paintball Mask - Blaster White

- Tummy Control Underwear High Waisted Cotton Soft Breathable Stretch Regular Cheeky Hipster Blue S
- Choosing the Ideal Men's Swimwear to Suit Your Body Type – TIMOTEO

- CK One Red Edition for Her Calvin Klein perfume - a fragrance for

- Gymshark GLCT1847-WJM-XS Ladies Vital Seamless Long Sleeve Crop
