In the following compressibility factor (Z) vs. pressure graph 300

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement
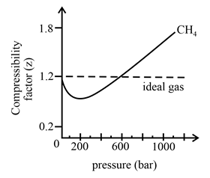
In the following compressibility factor Zvs pressure graph at 300Kthe compressibility of CH4 at pressures 200bardeviates from ideal behaviour because

Compressibility factor of water vapor along its saturation curve. Error
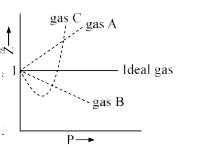
Gas C is a real gas and we can find 'a' and 'b' if intersection data i
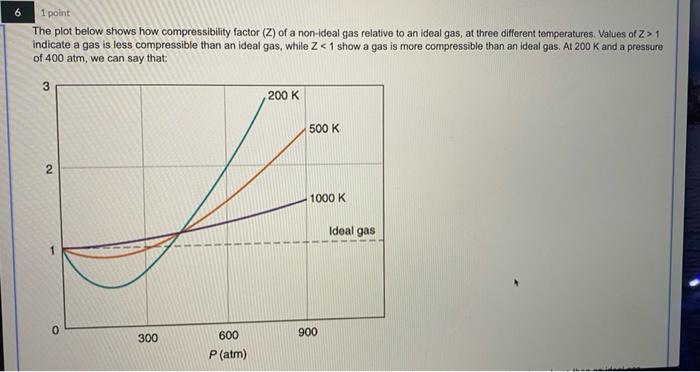
Solved 6 1 point The plot below shows how compressibility

Compressibility factor Z as function of temperature T with lines of
Speed of sound in hydrogen isotopes derived from the experimental pvt data and an improved quantum law of corresponding state

Energies, Free Full-Text

In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar

Compressibility Chart - an overview

In the following compressibility factor (Z) vs. pressure graph 300 K, the compressibility of CH_{4} pressure < 200 bar deviates from ideal behaviour becauseThe molar volume of CH_{4} is than its molar

Improved description of the liquid phase properties of Methane: density, enthalpy, plus saturated vapor compressibility factor
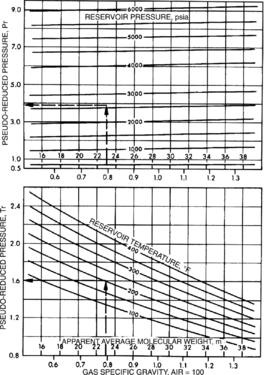
Static gas pressure gradient estimation - AAPG Wiki

gas laws - Graph of compressibility factor vs pressure when real gas is assigned Z=1 - Chemistry Stack Exchange
- physical chemistry - Is the compressibility factor smaller or

- Chapter 3 - Physical Properties of Fluids: Gas Compressibility

- Gas Z Factor Calculator: Dranchuk-Abou-Kassem · PVT Solver

- Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
- gas laws - How to find the temperature relationship between the

- Spanx Undie-tectable Lace Hi-Hipster Panty Very Black

- SlidesCarnival: Free PowerPoint & Google Slides Templates That

- Catherines Tank Top Cami PLUS 1X 18/20W Green Layering Shirt

- The Wiggles' Wiggle Groove Tour comes to Wellington - WellingtonNZ
- Gray Full Zip Up LuLu Style Atheletica Scuba Workout Casual Everyday Jacket - Veg4U
