What is the compressibility factor (Z) for 0.02 mole of a van der Waal

By A Mystery Man Writer
(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

6.3: Van der Waals and Other Gases - Physics LibreTexts
Energies, Free Full-Text

Van Der Waals Equation - an overview

SOLVED: The van der Waals constants for SO2 are a = 6.775 atm L^2

Physical Chemistry The Compression Factor (Z) [w/1 example

0.585%NaCl solution at 27∘C has osmotic pressure of

DPP No. : 21 Total Marks : 40 Max. Time : 10mln Single choice Objective (..

Thermodynamics: An Engineering Approach - 5th Edition - Part II by
4.3 – Non-Ideal Gas Laws — project1 1.0 documentation
58.7 Maximum mass of hydrogen is present in(1) 0.1 mol of CH1206(2) 1.5 mol of NH3(3) 22.4 L of H2S(g) at S.T.PSo(4) 0.5 g molecule of CeH

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20
- Math cad compressibility factor, z, of real gas using the redlich-kwong equation of state

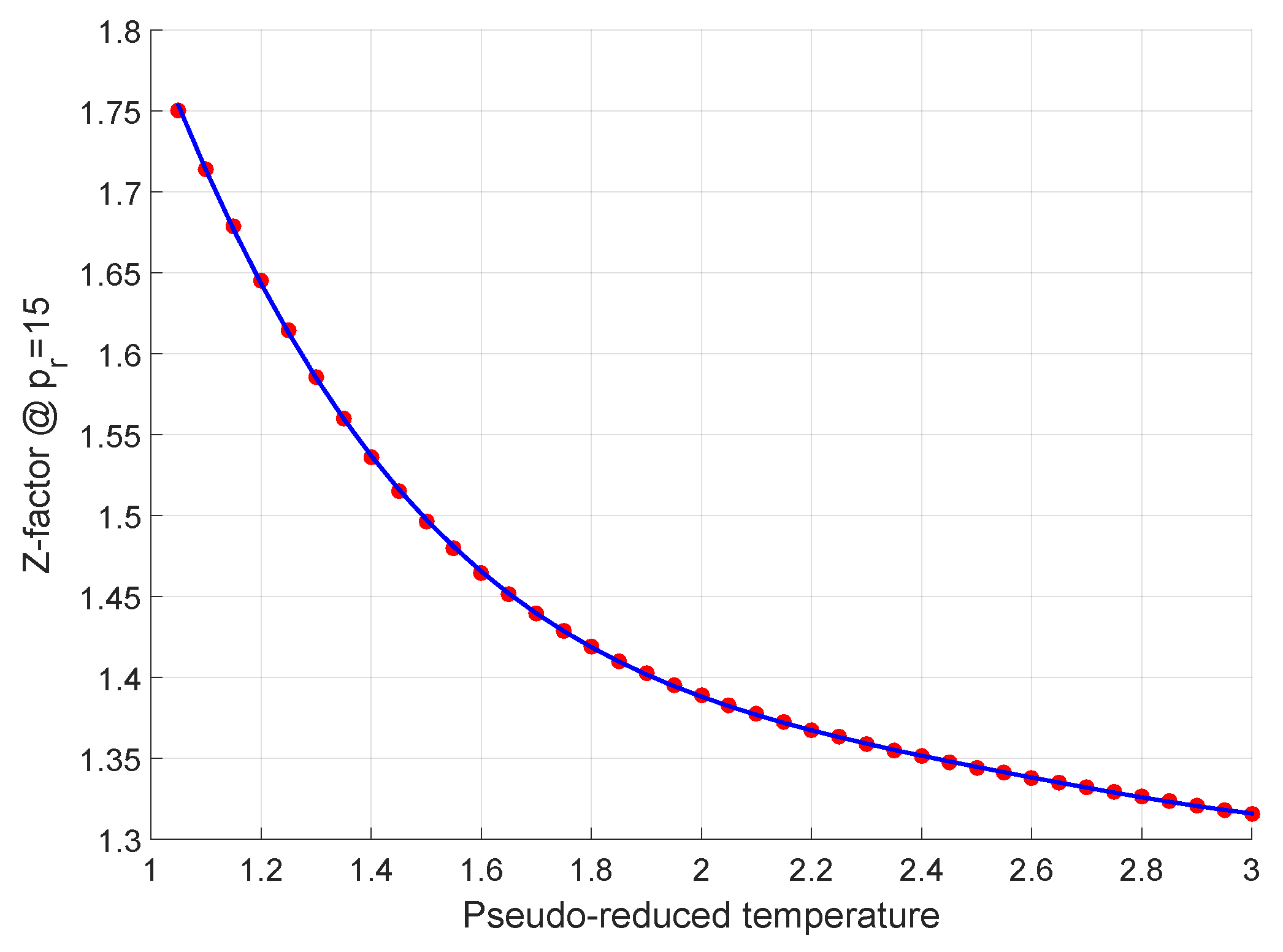
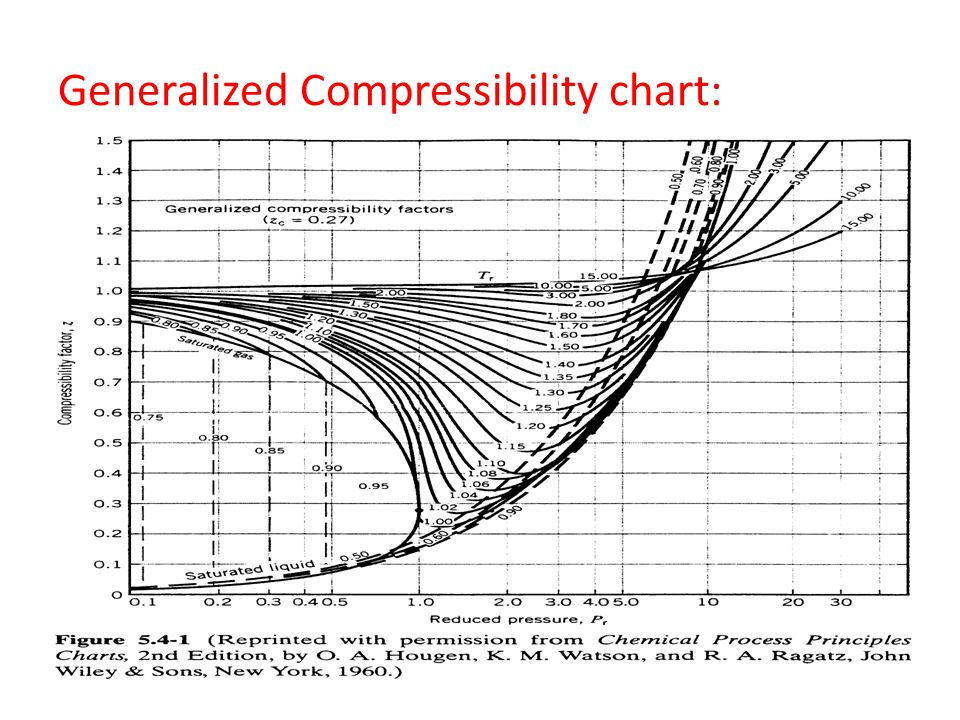
- 22 Generalized Compressibility Factor, Z

- Calculate the Compressibility Factor 'z' for Hydrocarbon Gases • zFactor

- Solved The compressibility factor, Z, can be thought of as a

- Solved The definition of compressibility factor Z, Eq.





