Solved The compressibility factor, Z, can be thought of as a

By A Mystery Man Writer
Answer to Solved The compressibility factor, Z, can be thought of as a

What is compressibility factor? What is its value for ideal gas

The value of compressibility factor (`Z`) for an ideal gas is
The value of compression factor at the critical state of a vander waals gas is
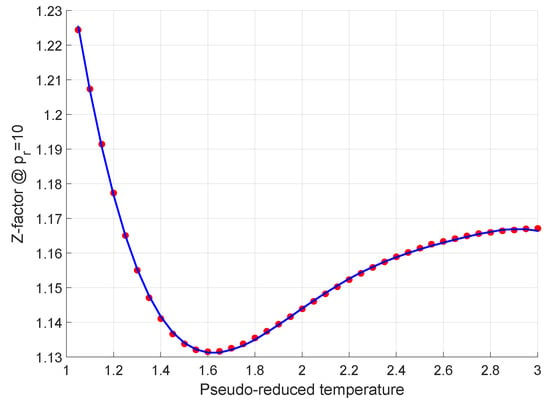
Energies, Free Full-Text
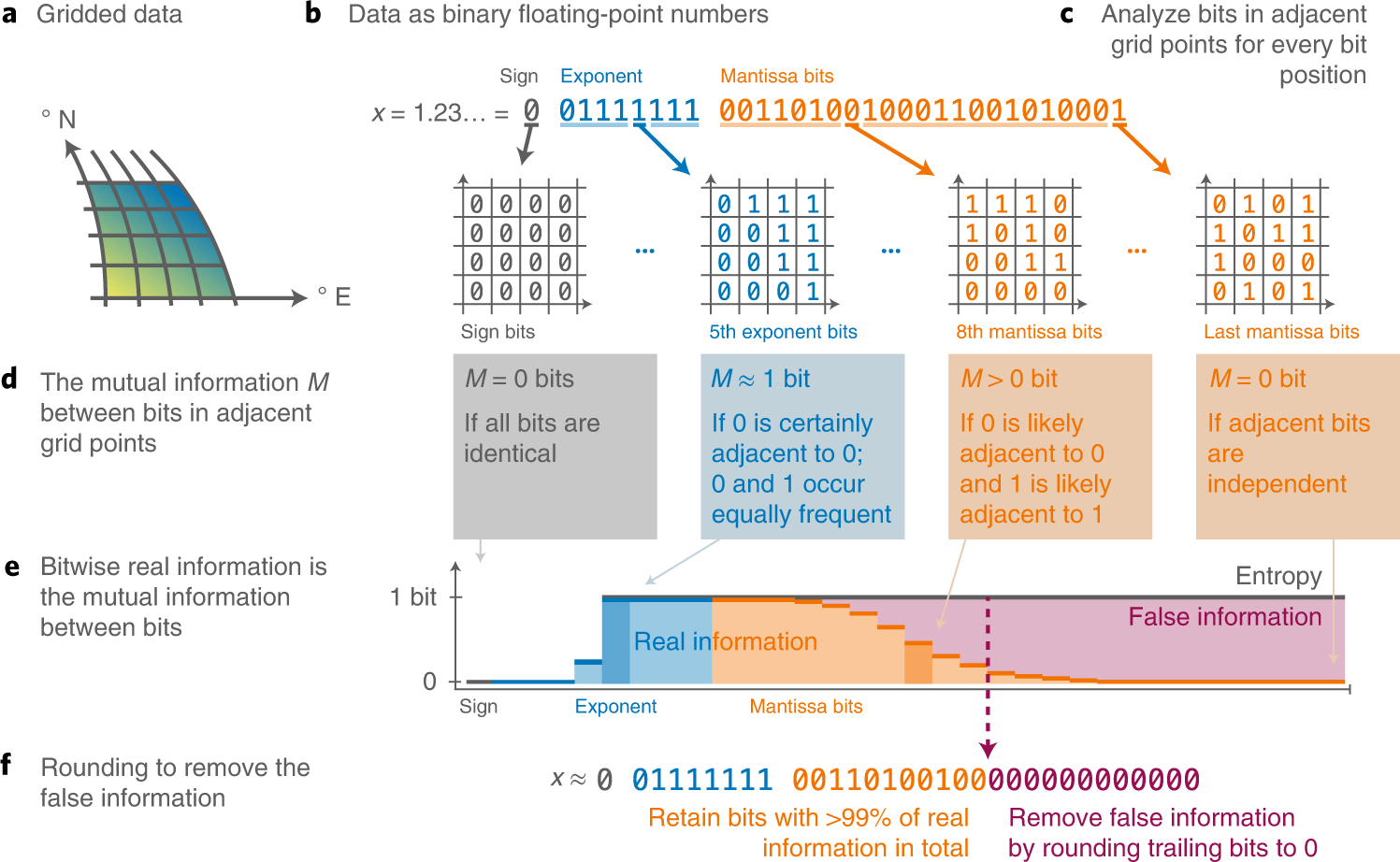
Compressing atmospheric data into its real information content

Determine Compressibility of Gases

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange
Evaluation of fugacity (Chemical Engineering)
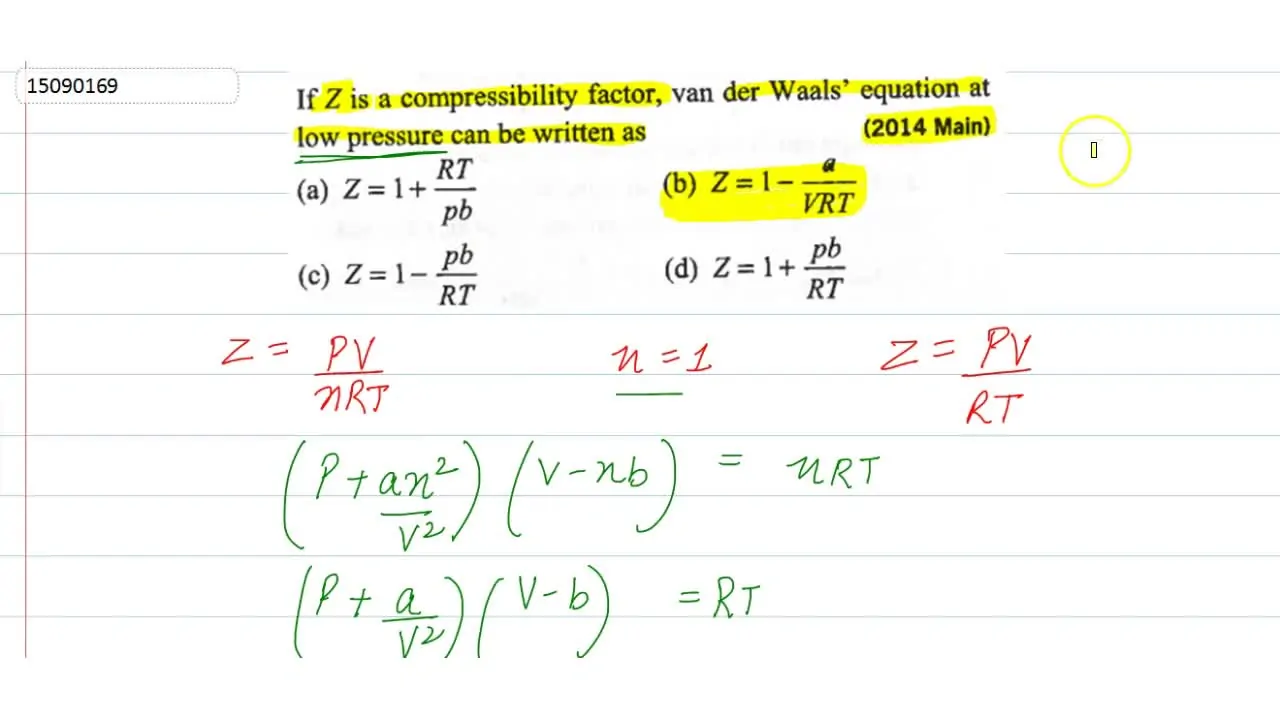
If Z is a compressibility factor, van der Waals' equation at low press
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

Energies, Free Full-Text

Compressibility factor - Wikipedia

The compressibility factor `(Z=PV//nRT)` for `N_(2)` at `223 K` and `81.06 MPa` is `1
- Explain how the compression factor varies with pressure and

- Super-critical Fluid Compressibility Factor Z , for Intermediate

- Air Compressibility Factor Table - EnggCyclopedia
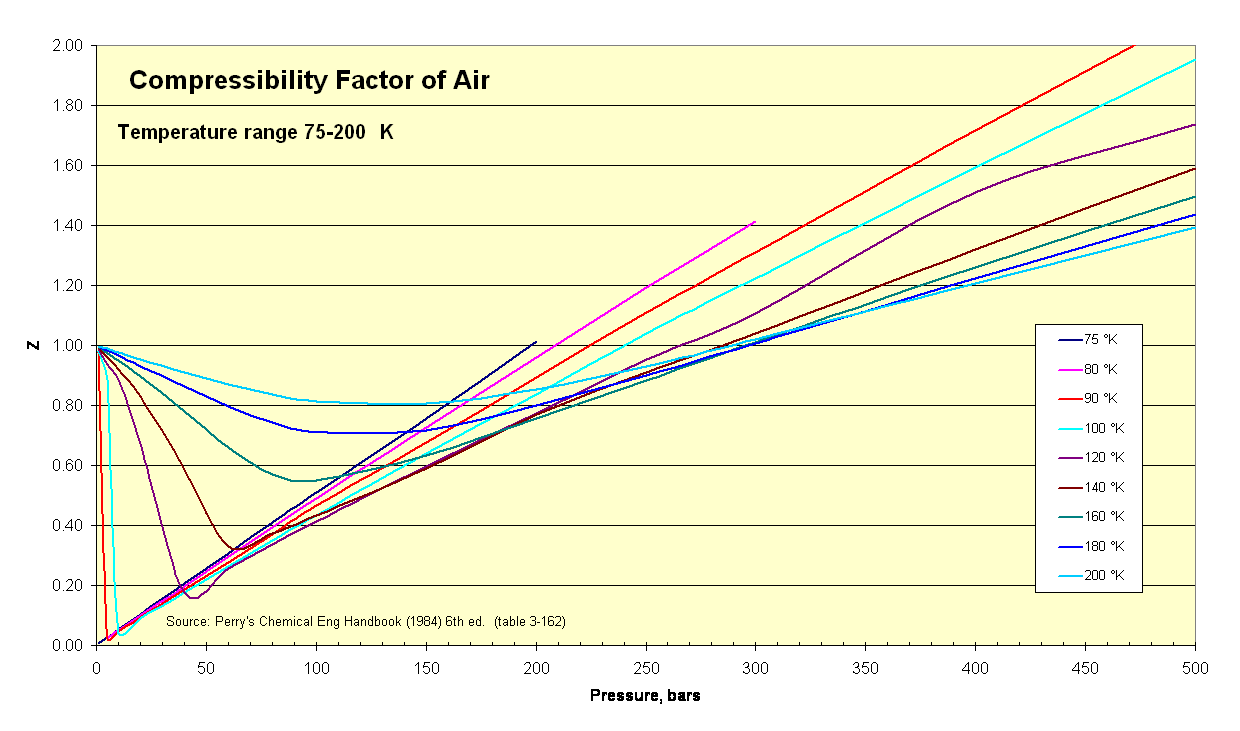
- Compressibility factor Z for sub-critical pressures in a 'one-cell

- PDF] Two Simple yet Accurate Equations for Calculating the Fugacity Coefficient Phi and the Gas Compressibility Factor

- Women's High Waist Joggers Outdoor Hiking Cargo Pants Khaki

- Falling Off Cliff Stock Illustrations – 134 Falling Off Cliff Stock Illustrations, Vectors & Clipart - Dreamstime

- FINETOO 10 Pack Thongs for Women Cotton Underwear V Philippines

- The Number Ones: Katy Perry's “California Gurls” (Feat. Snoop Dogg)

- Men's 100% Merino Wool Boxer Brief Underwear
