Compressibility factor (Z) for a van der Waals real gas at critical point is

By A Mystery Man Writer
Share your videos with friends, family and the world

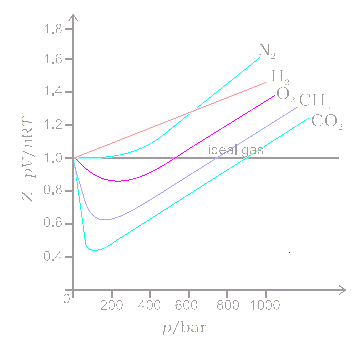
Determine Compressibility of Gases
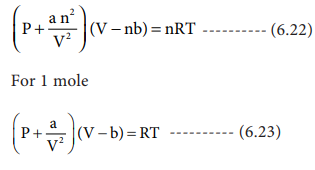
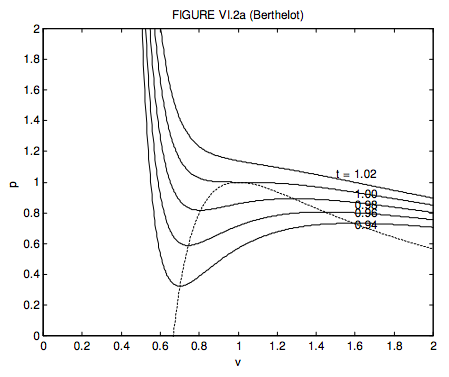
Derivation of critical constants from van der Waals constant

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is
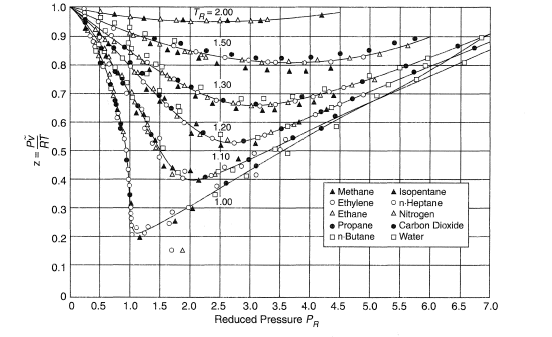
GAS LAW
How can we calculate critical temperature, volume and pressure in terms of a and b? - Quora
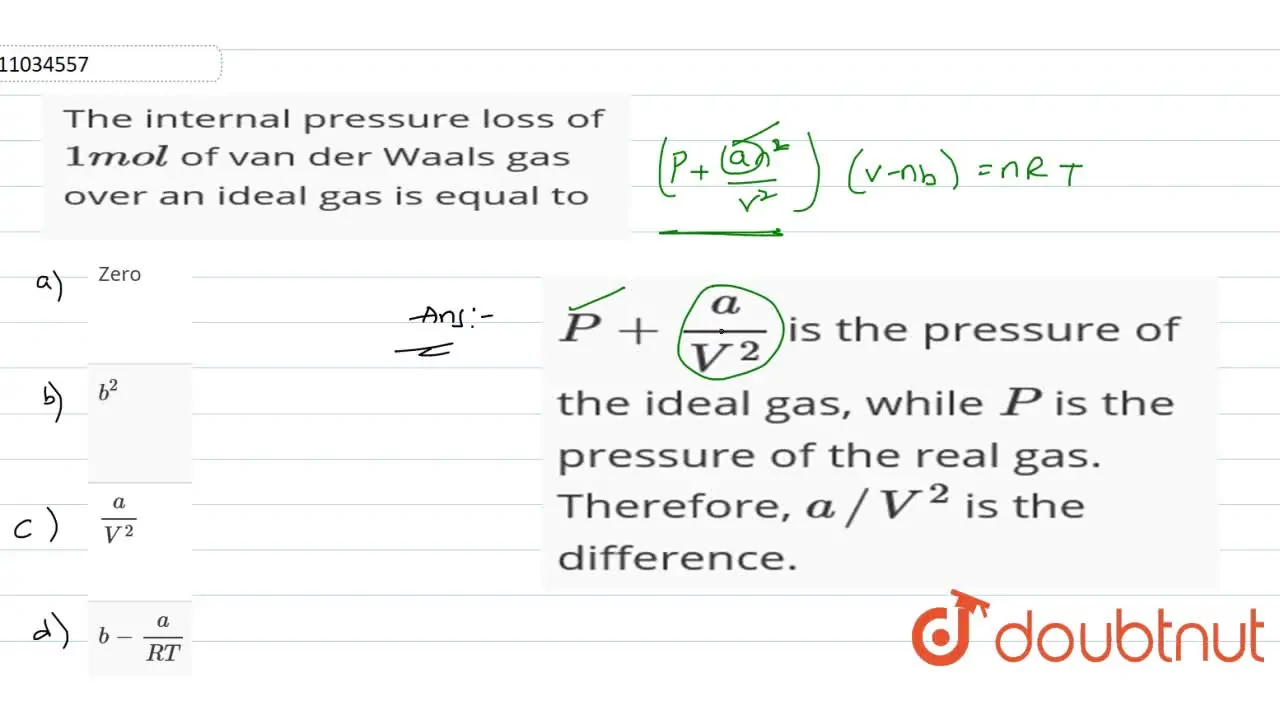
The internal pressure loss of 1 mol of van der Waals gas over an ideal
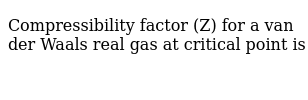
Compressibility factor (Z) for a van der Waals real gas at critical po
6.3: Van der Waals and Other Gases - Physics LibreTexts
Van der waals equation: Derivation, Explanation
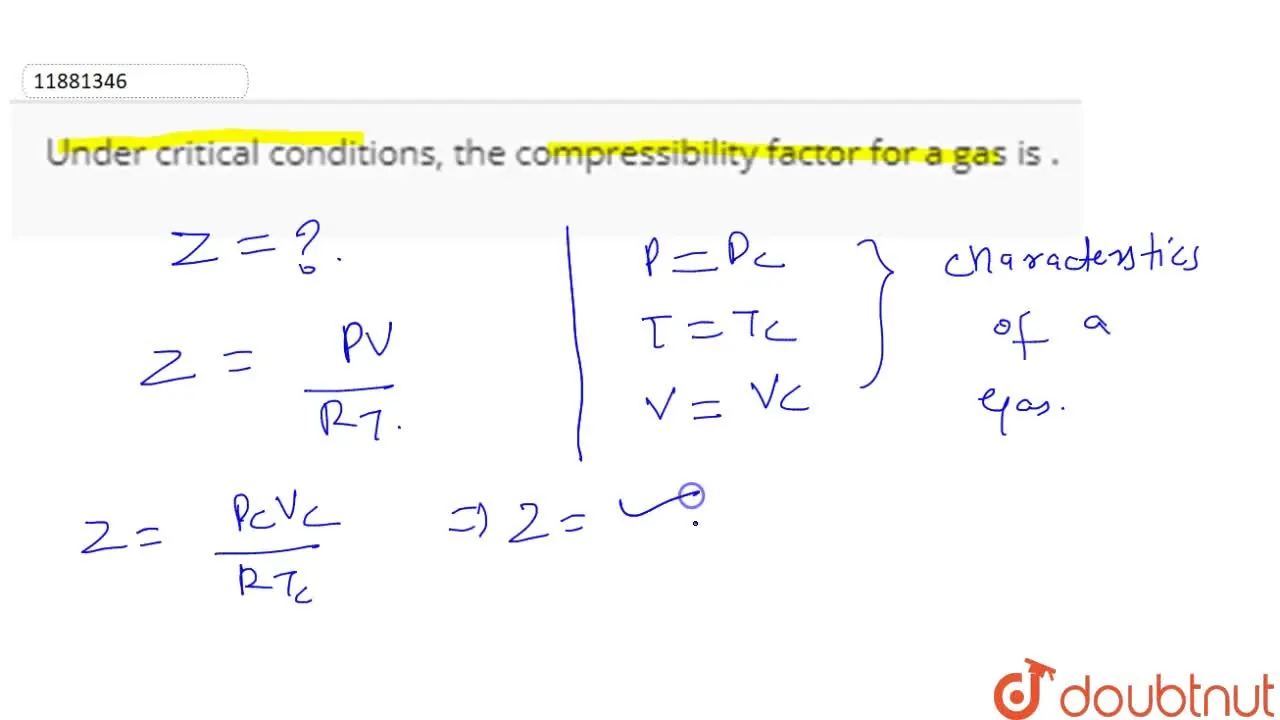
Under critical conditions, the compressibility factor for a gas is .

PDF) Critical State Behavior of Van der Waal gases Conformation to Nelson Obert Characteristics
For a certain van der Waal's gas, critical temperature is-243^(@)C. Ma
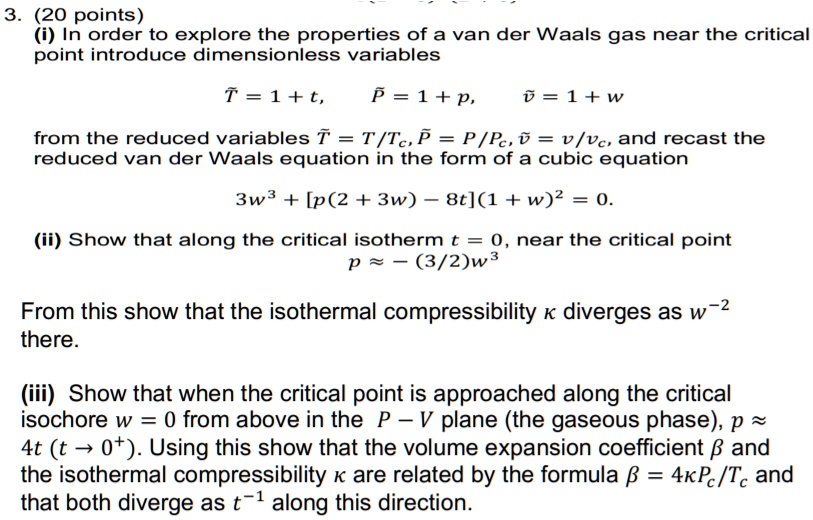
SOLVED: (i) In order to explore the properties of a van der Waals gas near the critical point, introduce dimensionless variables: T = 1 + t, P = 1 + p, V =

Compressibility factor (Z) for a van der Waals real gas at critical point is

Fluids, Free Full-Text
- Cubic Equation of State for the Compressibility Factor - Wolfram Demonstrations Project
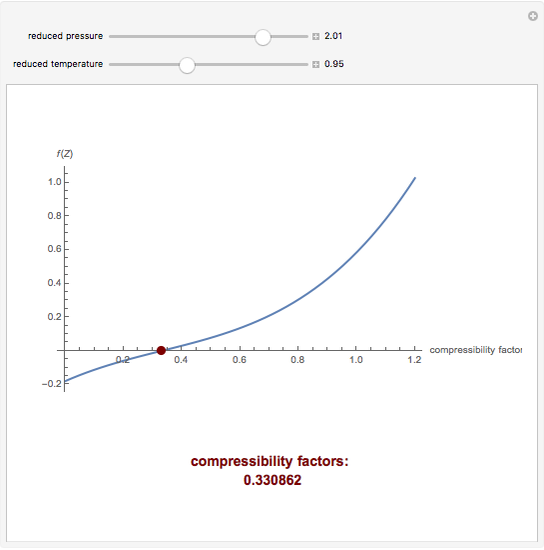
- Solved We showed, for a van der Waals gas, that the
- The compressibility factor is Z = PV/R_g T. Evaluate

- 3.3.3: Natural Gas Properties PNG 301: Introduction to Petroleum and Natural Gas Engineering
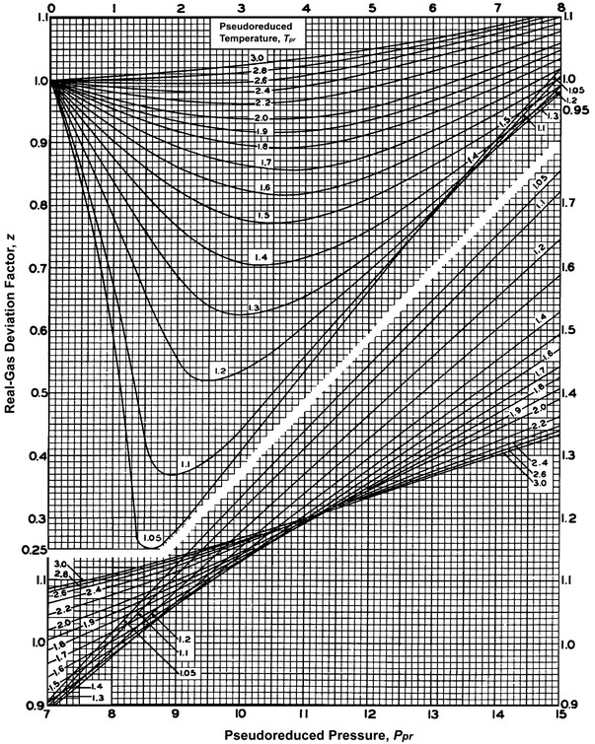
- The compressibility factor Z for an ideal gas will be

- Style & Co Plus Size High Rise Capri Leggings, Created for Macy's

- KosmoCare: Health & Personal Care

- Denver Hayes Men's Stretch Long Sleeve Modern Fit Crewneck T Shirt

- SYROKAN Women's Sports Bra High Impact Wire Free Lightly Padded Full Coverage – ASA College: Florida

- Top 5 Best Sexy Active Wear Outfits - Look as Sexy as You Feel - Shape Brazil

