The compressibility factor Z for an ideal gas will be

By A Mystery Man Writer
The compressibility factor Z for an ideal gas will be

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

Select the incorrect statement:aCompressibility factor foran ideal gas is unity.A real gas approachesideal

Compressibility factor for methane.

Question No 5 4 Digit Integer Type Question Q.1 to Q.6 are
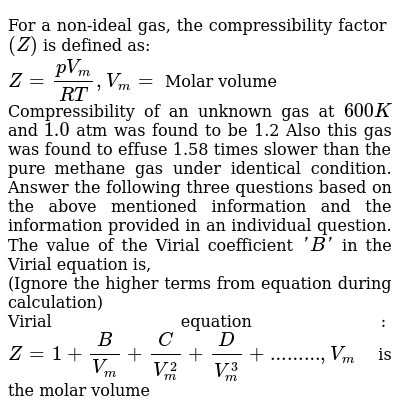
For a non-ideal gas, the compressibility factor (Z) is defined as: Z

2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

The given graph represent the variations of Z Compressibility factor Z PV nRT versus p for three real gases A B and C Identify the only incorrect statement
The compressibility factor for an ideal gas is
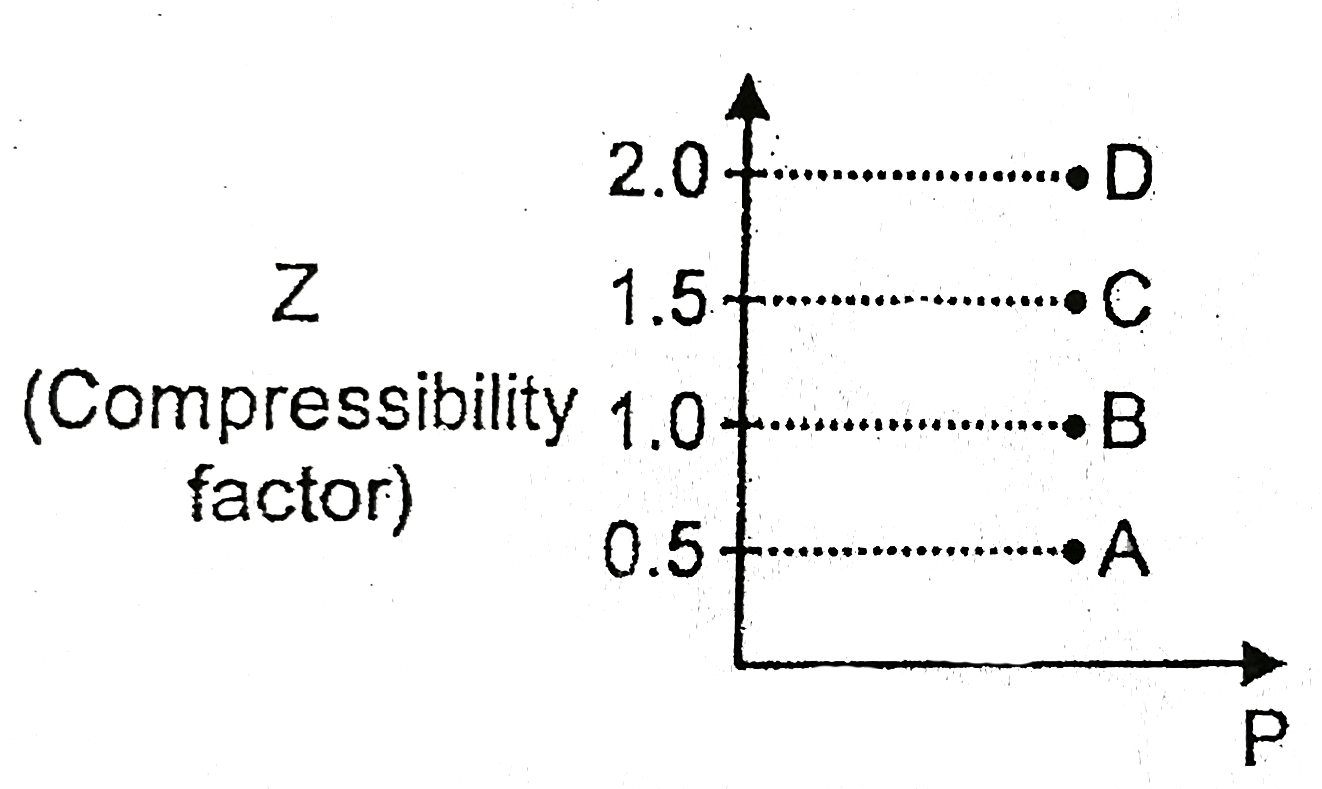
Z is less than 1 and repulsive forces are dominant.
- Ideal Gas Equation and COMPRESSIBILITY Factor in 11 Minutes!

- PPT - GASES PowerPoint Presentation, free download - ID:2088317

- Equation of state (excess compressibility factor, Z À1 ¼ PV/(NkT) À1

- 20.If Z is a compressibility factor, van der Waals equation at low
- Thermodynamic Properties Property Table w Property Table -- from

- Women Plunging Deep V-neck Body Shaper Strapless Backless Bodysuit

- Nike Swoosh Women's Training Sports Bra - Black/White

- Marc New York Women's Performance Capri Cropped Pants Size 2X

- Sujetador de corsé de sirena de resina de escamas de sirena de cristal, disfraz de Cosplay superior, protegido de patente

- Flicarts Women's Transparent Backless Strapless Invisible Clear Back Underwired Padded Push Up Bra(Sky-Blue)
