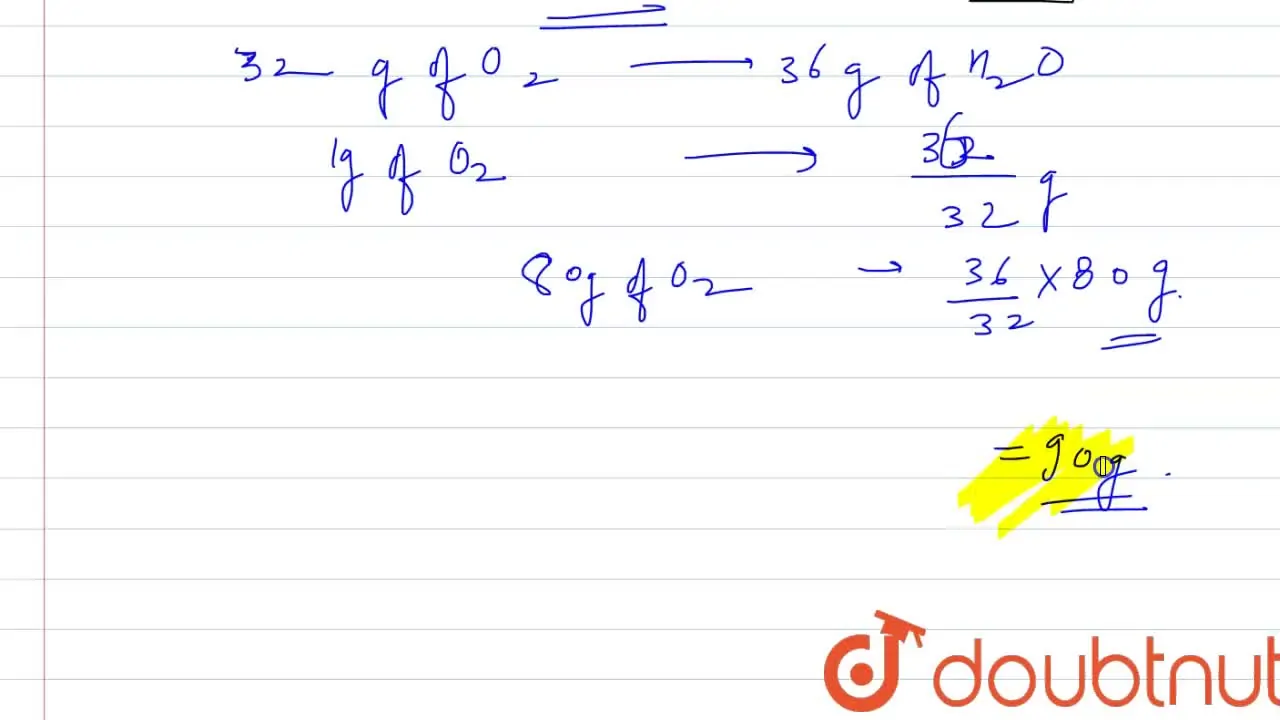
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out
By A Mystery Man Writer
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent
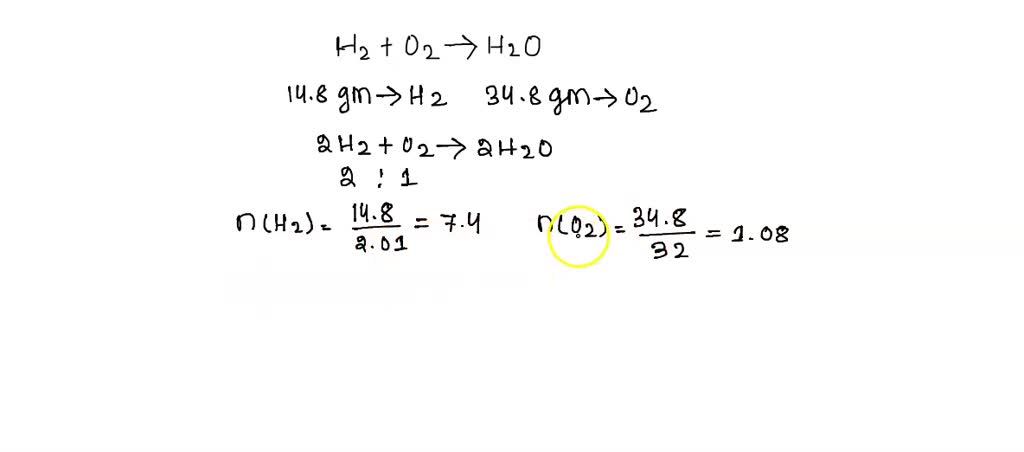
SOLVED: Question 5 Not yet answered Marked out of 1.00 Flag

Q. 88.6 mathrm{g} of mathrm{H}_{2} reacts with 32 mathrm{g} of mathrm{O}_{2} to yield water. Which is the limiting reactant? Find the mass of water produced and the amount of excess reagent left.
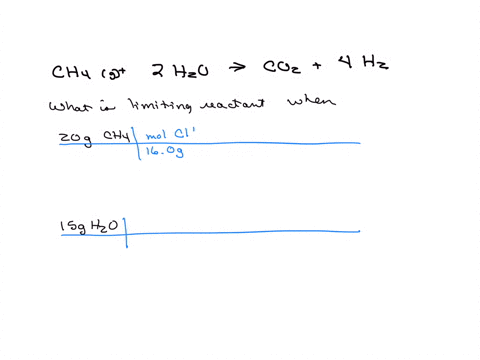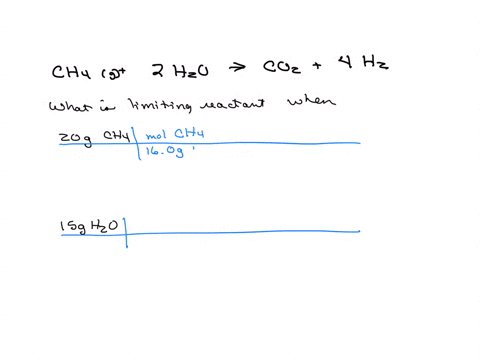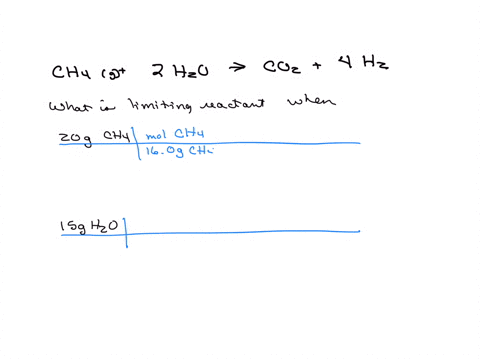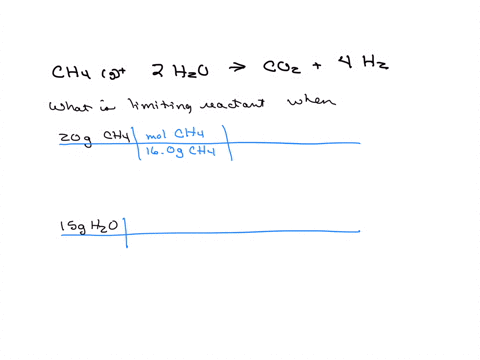
SOLVED: Question 1: CH4 + 2 H2O → 4 H2 + CO2 Given 80 g of CH4

Catalysts, Free Full-Text
80 g of H(2) is reacted with 80 g of O(2) to form water. Find out the

Photocatalytic H2 evolution and apparent quantum yield of Pt
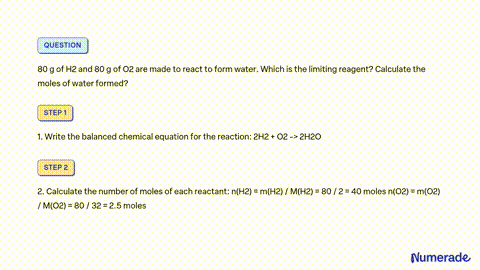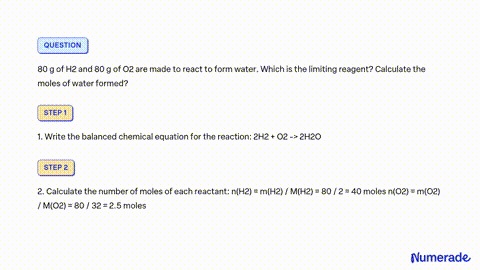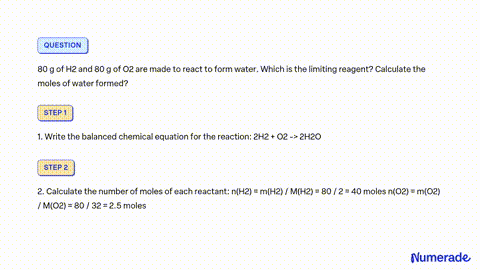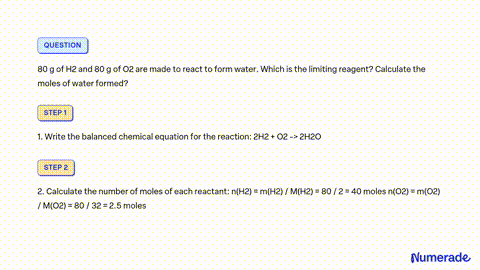
SOLVED: A mixture containing 64 g of H2 and 64 g of O2 is ignited
How many grams of water can be produced if sufficient hydrogen
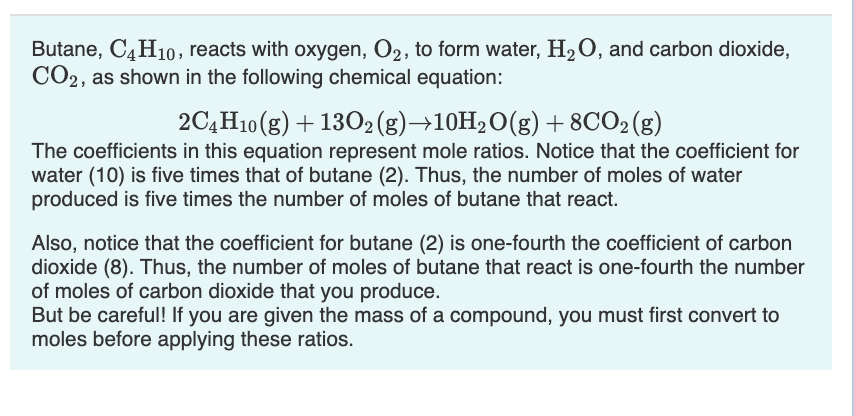
Solved Butane, C4H10, reacts with oxygen, O2, to form water
How much mass of water is obtained by reacting 80 g each of

Solved Hydrogen gas and oxygen gas react to form water. The

Malayalam] Find out the limiting reagent when 5g of H2 reacts with 24
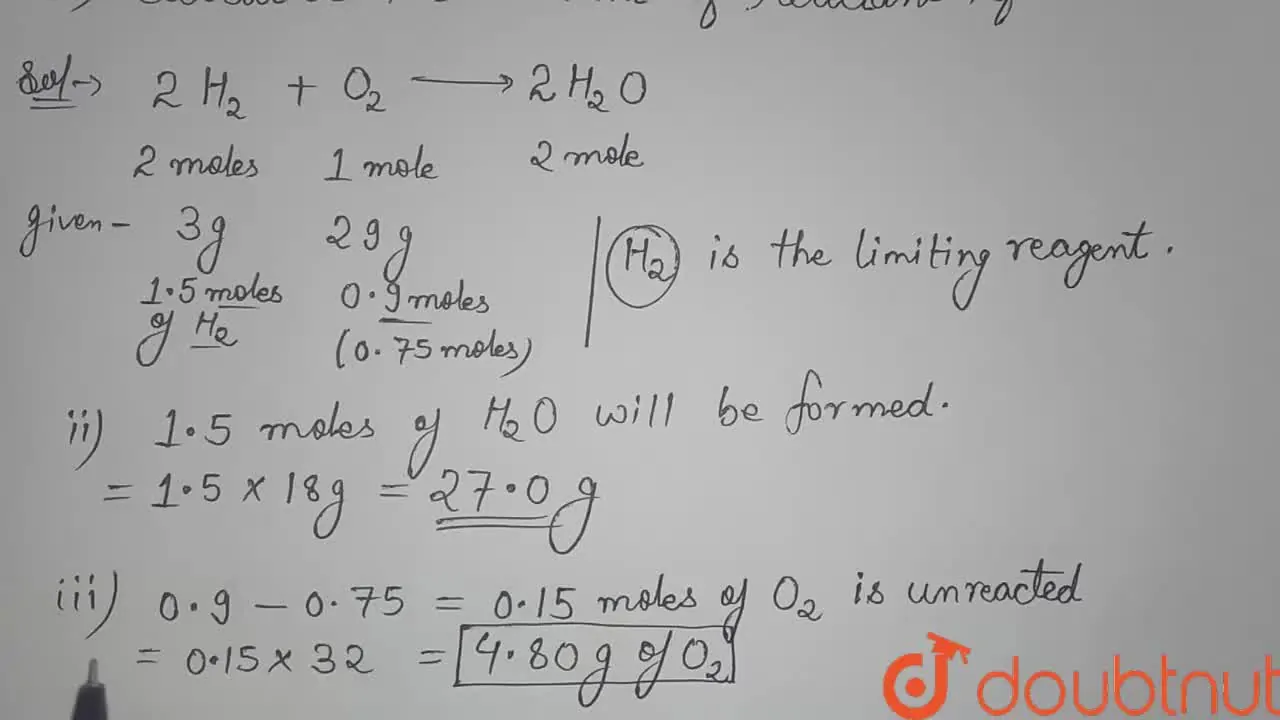
3.0 g of H(2) react with 29.0 g of O(2) yield H(2)O. (i) Which
- Fralda Looping Looney Tunes Mega G 32 Unidades - Drogaria Venancio
- Smartphone Motorola Moto G20 Dual SIM 64 GB pink 4 GB RAM

- Pepero Palitinhos c/ Chocolate Choco Cookie 32g - HARU PRODUTOS ORIENTAIS E NATURAIS

- Moto G5 Plus Dual SIM 32 GB cinza-lunar 2 GB RAM XT1683 - MOTOROLA

- Fralda BabySec Ultra Mega 6 Pacotes Tamanho G - 32 Unidades Cada - Fralda Descartável - Magazine Luiza

- NWT Alo Yoga Ribbed Dusty Pink Muse Sweatpants Large $98 Selling Matching Top

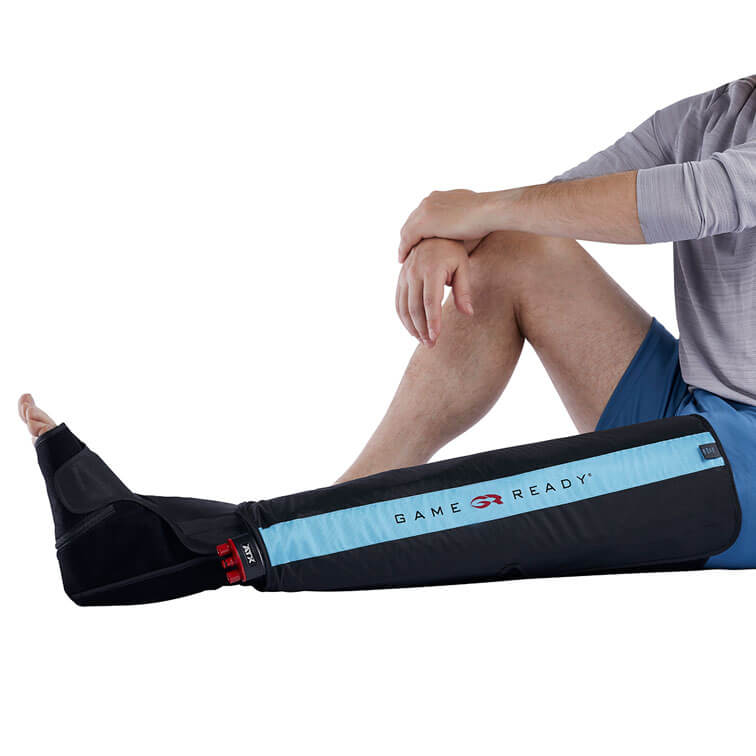
- Game Ready Wraps, Physical Therapy Equipment
- 10 Inspirasi OOTD Jalan-jalan Pakai Tank Top ala Kapook

- Bobby Brazier's home with lookalike dad Jeff Brazier is a boho dream – see photos

- X-2 Men Track Suits 2 Pieces Set Full Zip Sweatsuit Men Hooded Tracksuit Athletic Sports Set Teal Blue X-Large
