PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis

By A Mystery Man Writer
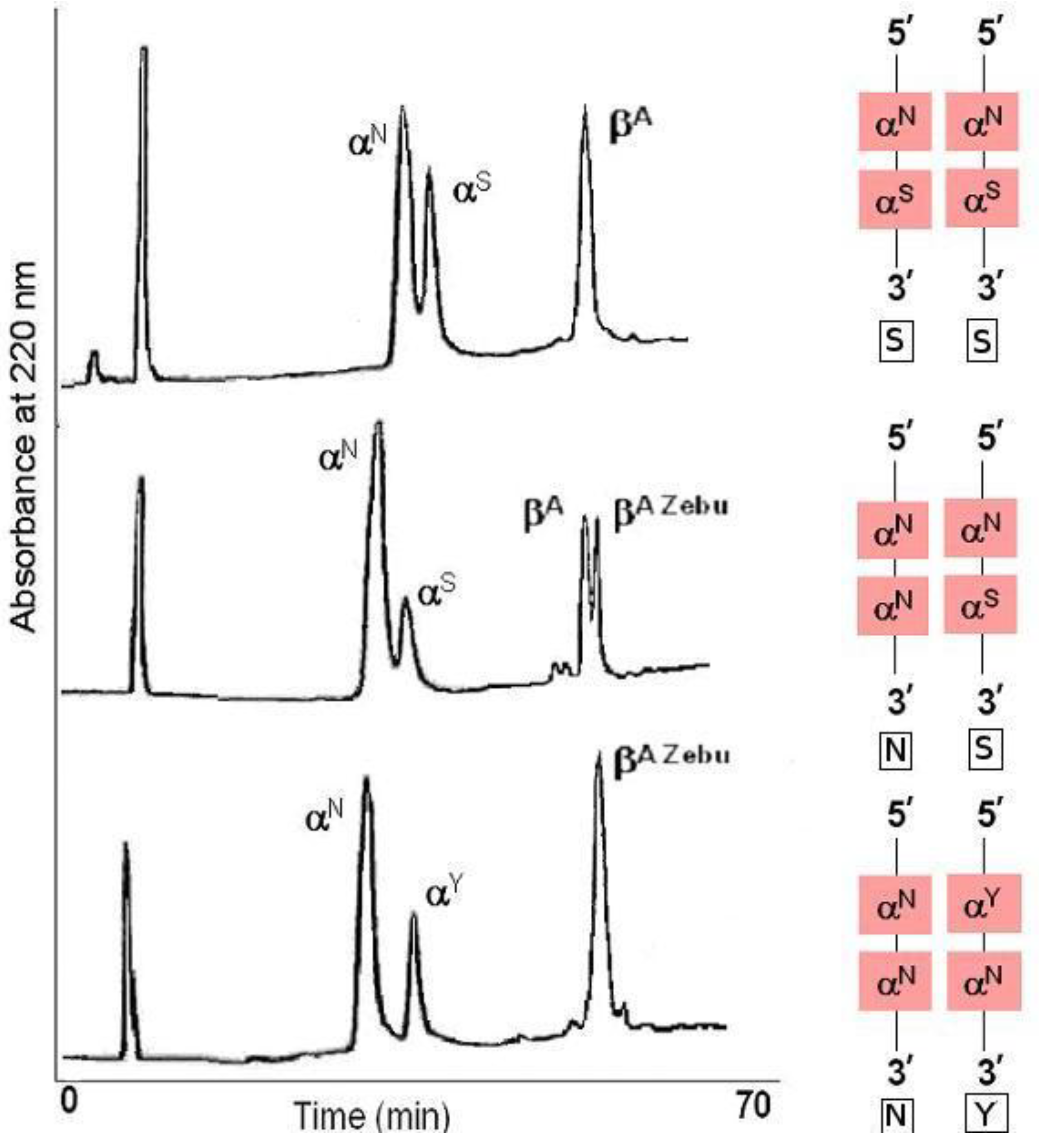
It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains in white-tailed deer. A variety of aberrant erythrocyte forms have been related to seven adult and two fetal hemoglobins in white-tailed deer. While sickling of the erythrocyte was not associated with a single hemoglobin type, it was precluded by hemoglobin V or VII, even when in combination with other hemoglobin types normally associated with sickling. The subunit basis of the hemoglobin polymorphism was presented. Two kinds of α subunits, six kinds of β subunits and one γ subunit were related to the whole hemoglobin molecule. The heterogeneity of the deer hemoglobins was based upon a variety of combinations of these numerous polypeptide chains. It was concluded from the results of limited structural studies that there were multiple peptide differences upon comparison of three non-α polypeptide chains.

Ultrastructure of Sickled Deer Erythrocytes. I. The Typical

(PDF) Pan-American Trypanosoma (Megatrypanum) trinaperronei n
Diversity, Free Full-Text
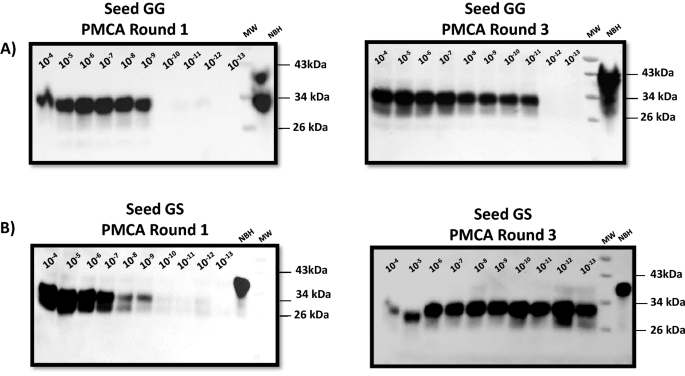
Chronic wasting disease (CWD) prion detection in blood from pre

The conserved Phe GH5 of importance for hemoglobin intersubunit
Diversity, Free Full-Text

Frontiers A New Homotetramer Hemoglobin in the Pulmonary

Genome-wide analysis reveals adaptation to high altitudes in
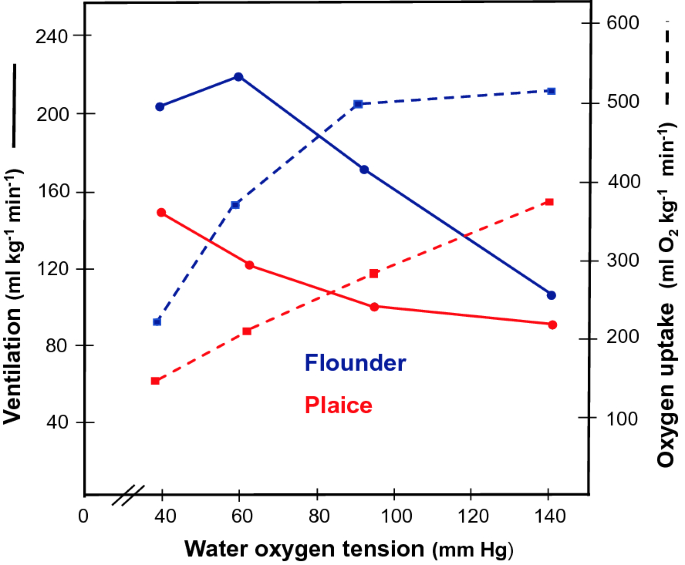
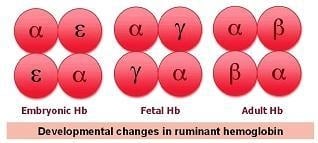
Multiplicity and Polymorphism of Fish Hemoglobins

PDF] Hemoglobin polymorphism in white-tailed deer: subunit basis

35, PDF

Relaxed functional constraints on triplicate α-globin gene in the
CoRIS Coral Glossary - NOAA's Coral Reef Conservation Program
Alteration of the α1β2/α2β1 subunit interface contributes to the

PDF) Gene Flow in the Face of Countervailing Selection: Adaptation
- HEMO Shapewear Women's Tummy Control Shapewear Light Satin Briefs

- HEMO Shapewear Women's Tummy Control Shapewear Plus Size Shorts

- HEMO Shapewear Women's Tummy Control Full Body Shaper Butt Lifter Seamless Bodysuit Tummy Control Shapewear Women Belt Butt Lifter Underwear Corsage (Color : Beige, Size : X-Large) : : Fashion

- estamos listas para atenderlas Hermosa😍🍑💪 @DM shapewear @DM SHAPEWE
- Aaron Goodman - “Papa Heme” on X: Hemoglobin Dissociation Curve! I have to teach this soon and was reviewing hemoglobin dissociation curve and came across this fantastic slide from The Calgary Guide