At low pressure, the van der waal's equation is written as (P+ a/V
By A Mystery Man Writer
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

At high temperature and low pressure, the van der Waals' equation

Why do we use the ideal gas equation when instead van der Waals
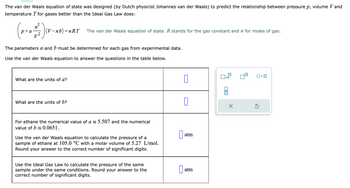
Answered: The van der Waals equation of state was…

Q.4 The van der Waals' equation for a gas is (p+V2a)(V−b)=nRT
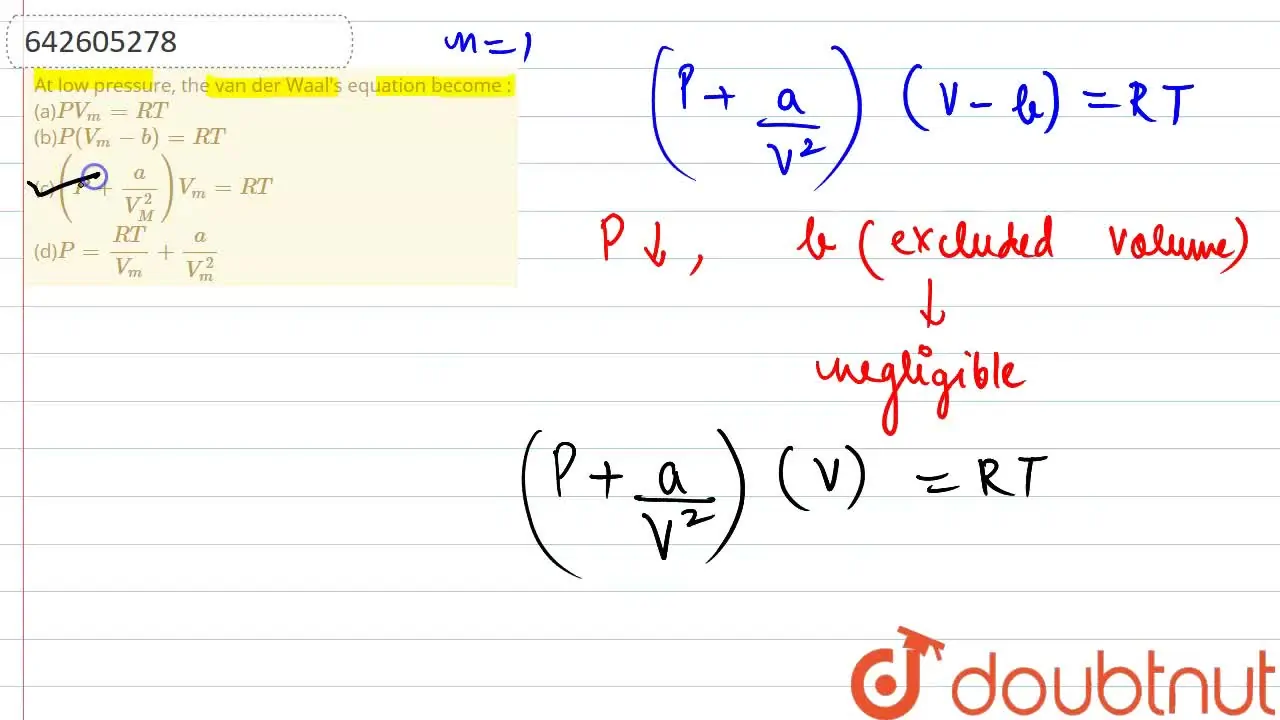
At low pressure, the van der Waal's equation become : (a)PV(m)=RT (b
p+a/v)(v b) =RT, p=pressure,v=volume,R,a,b are constant, T

A Quick Guide on Van der Waals Equation

The Van Der Waals Equation, PDF, Gases
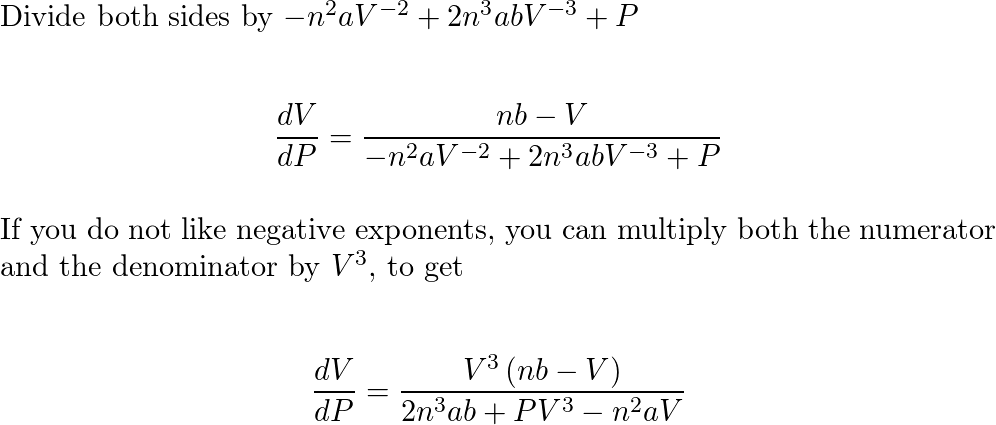
The van der Waals equation for n moles of a gas is $$ (P+n

a) The P -v diagram according to the Van der Waals Equation (Eq
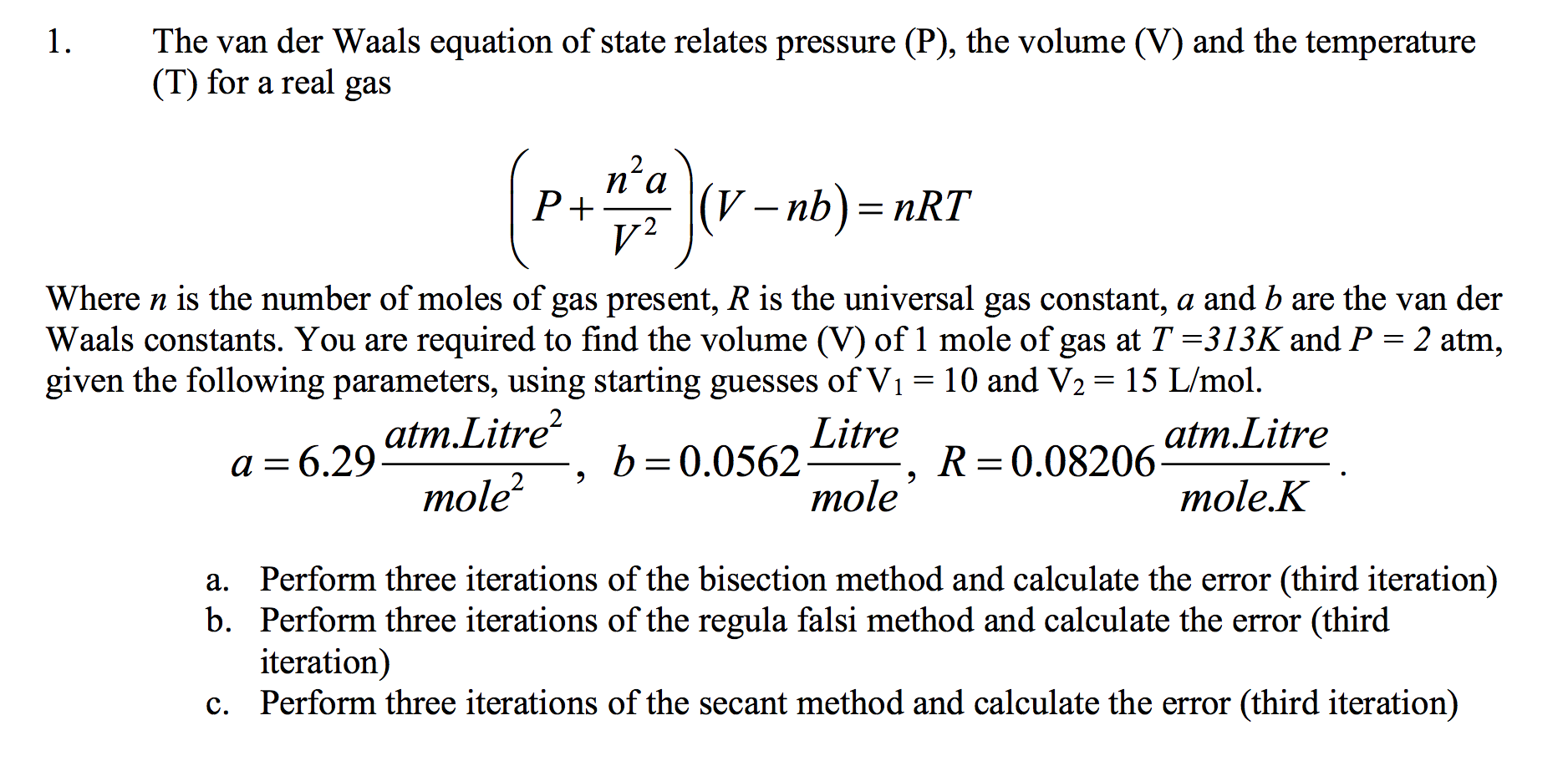
Solved 1. The van der Waals equation of state relates

Van Der Waals Equation: Overview, Questions, Easy Tricks, Rules
The equation of state for real gas is given by (P+a/V2)(V b)=RT

At low pressure, the compressibility factor is given as (1) RIV RTV RT

The Van der Waals equation a real gas is : (P + 2) (V – b) = RT









