The graph of compressibility factor (Z) vs. P for one mole of a real gas ..
By A Mystery Man Writer
Solution For The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very hi
The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very high pressure (dPdZ) is (2.81)atm−1 , then calculate volume of one mole of real gas molecules (in L/mol) Given : NA=6×1023 and R=27322.4LatmK−1mol−1

The graph of compressibility factor (Z) vs. P for one mole of a

For one mole of a real gas, curves are plotted under different
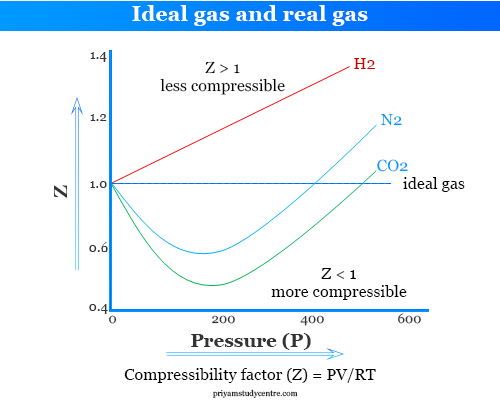
Ideal and Real Gases - Definition, Comparison, Properties

Compressibility factor (gases) - Citizendium

Deviation Of Real Gas From Ideal Gas Behavior
The given graph represent the variations of compressibility factor

11-06-23 - JR - Iit - Star Co-Sc (Model-A) - Jee Adv - 2014 (P-I

Level 3 Passage 3 from NARENDER AVASTHI CHEMISTRY (ENGLISH
In the following compressibility factor (Z) vs pressure graph at
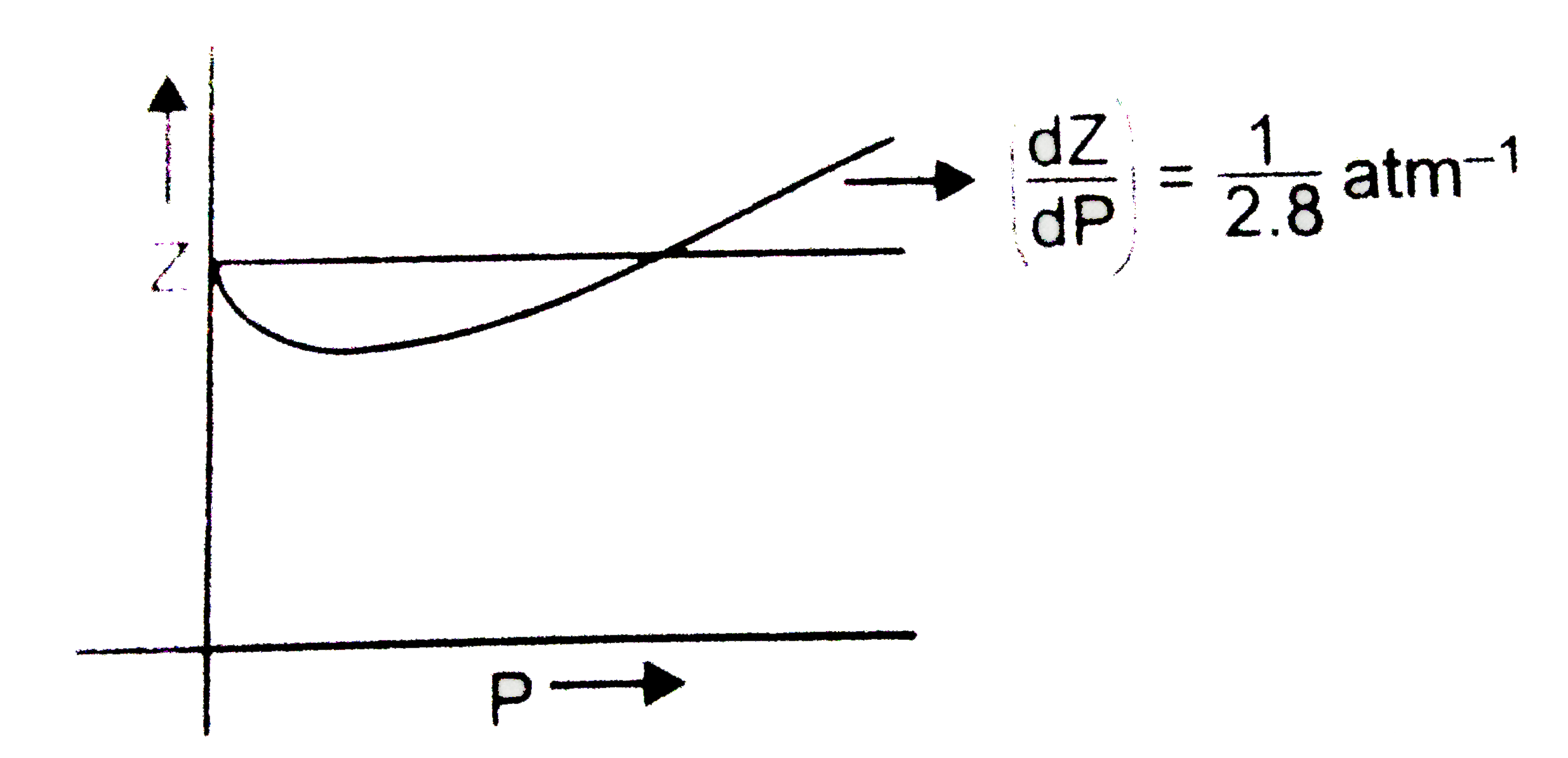
The graph of compressibility factor (Z) vs. P for one mole of a real g

The compressibility factor (Z) vs P one mole of a real gas is

The graph of compressibility factor (Z) vs. P for one mole of a real g

gas laws - Graph of compressibility factor vs pressure when real

Compressibility factor - Wikipedia
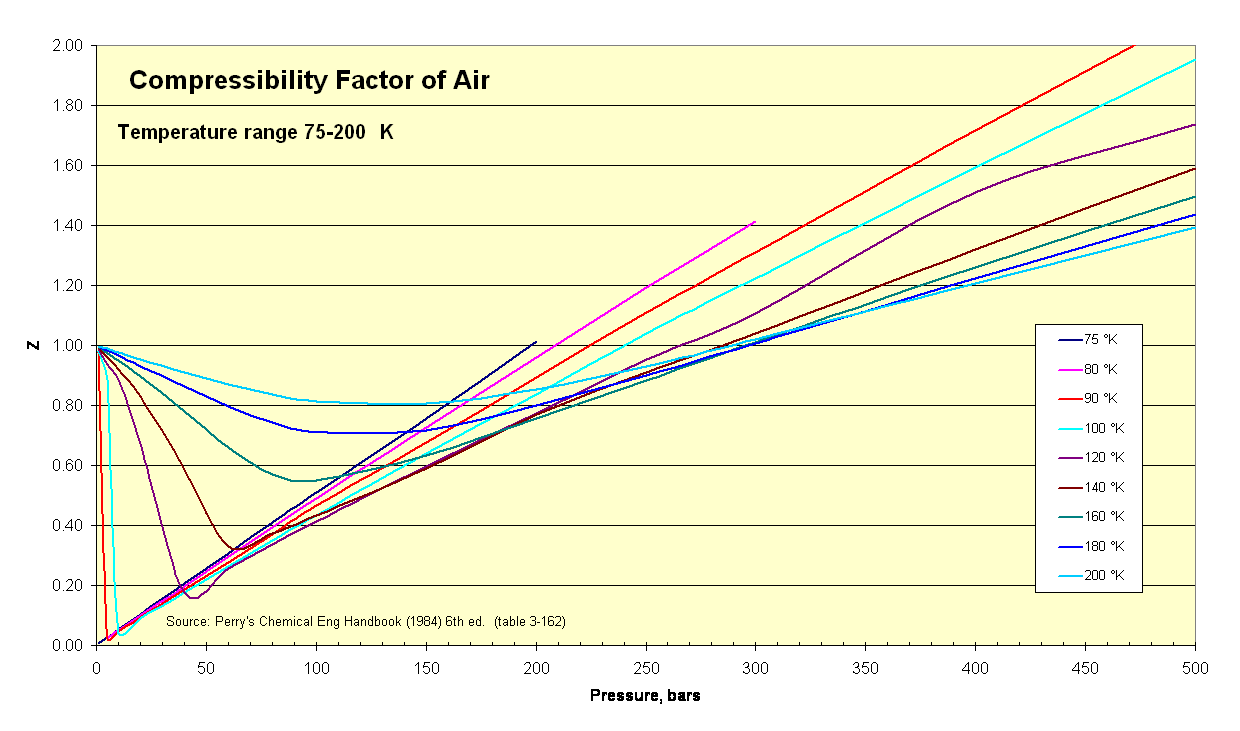
- Air Compressibility Factor Table - EnggCyclopedia
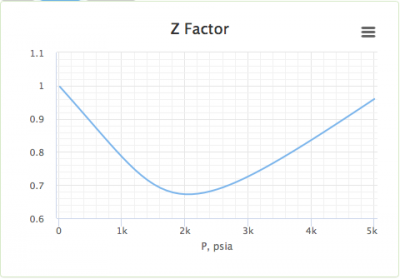
- Standing and Katz gas compressibility factor
- Compressibility factor Z for sub-critical pressures in a 'one-cell

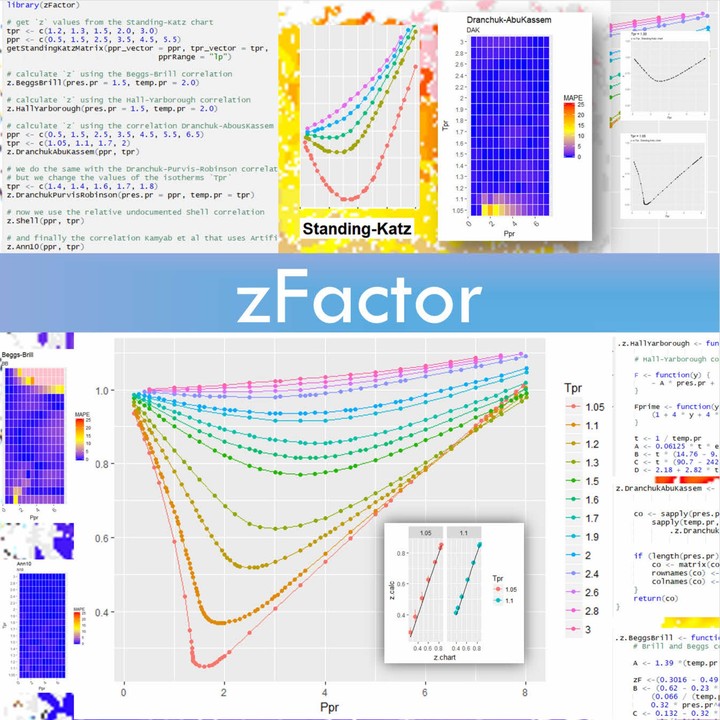
- Building your own petroleum engineering library with R: humble
- physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange





