For each of the negative ions listed in column 1, use the periodic table to find in column 2 the total number of electrons the ion contains. A given answer may be

By A Mystery Man Writer
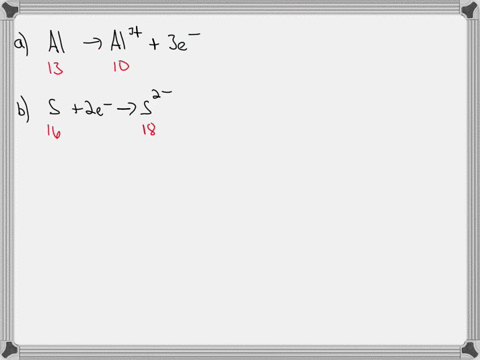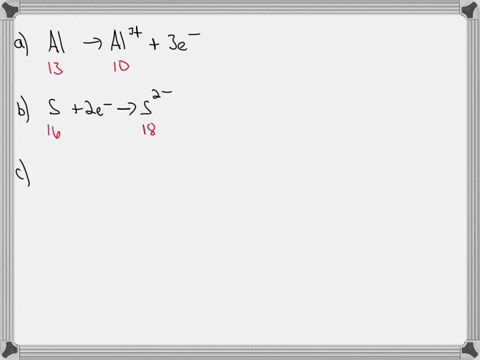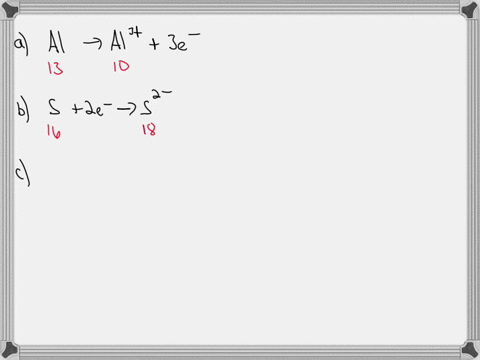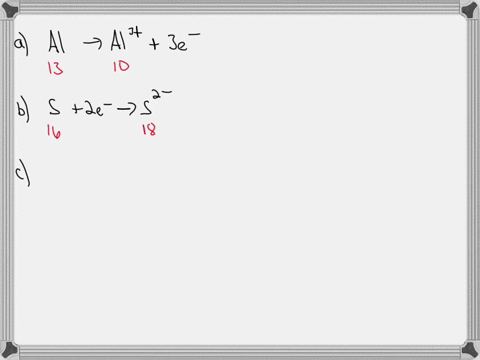
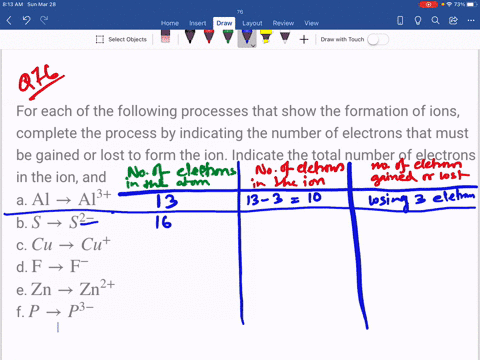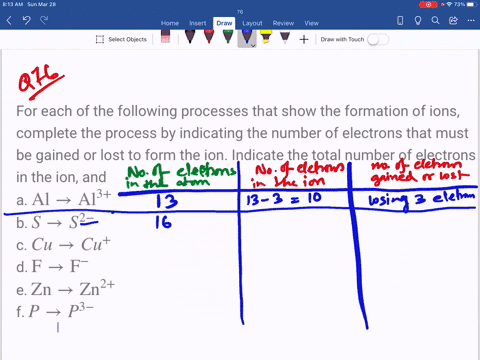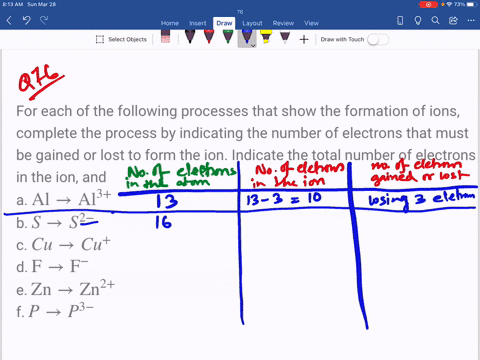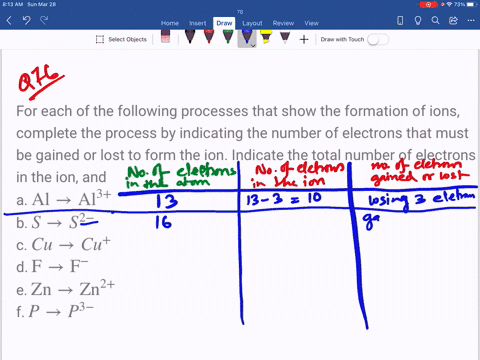
SOLVED: An ion is formed when an atom gains or loses an electron or electrons. Ions have a charge. If an atom has seven electrons in the outer shell, it will tend

SOLVED: Complete the third column (Number of Electrons in Ion) of the table. Express your answer as integers. Enter your answers in order given in the table, from top to bottom, separated
⏩SOLVED:For each of the negative ions listed in column 1, use the…

SOLVED: Complete the third column (Number of Electrons in Ion) of the table. Express your answer as integers. Enter your answers in order given in the table, from top to bottom, separated

Chapter 5, Nomenclature Video Solutions, Introductory Chemistry
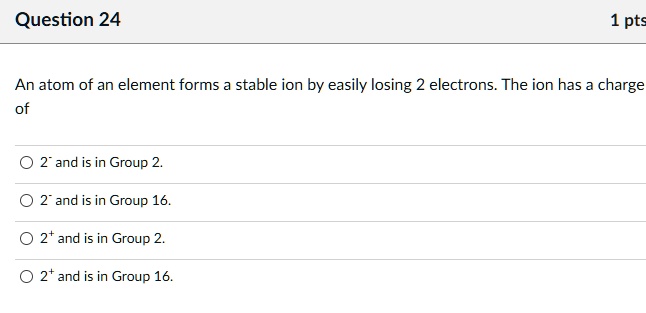
SOLVED: Question 24 1 pts An atom of an element forms stable ion by easily losing electrons. The ion has charge 2 and is in Group 2 2 and is in Group
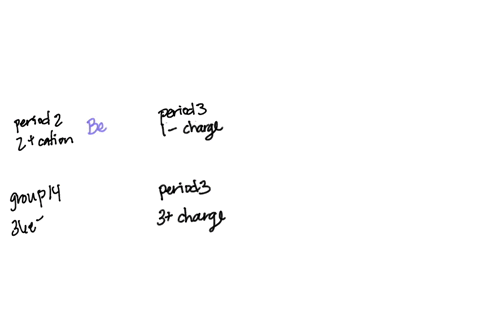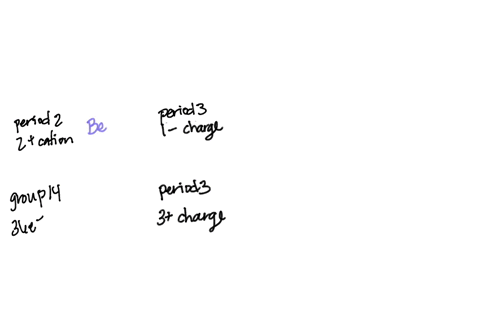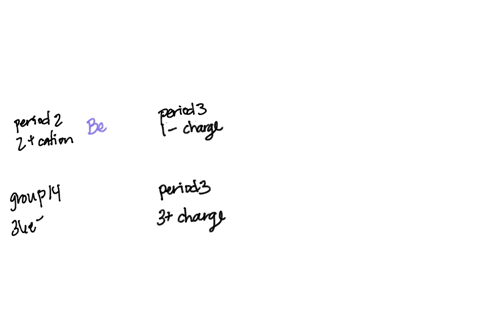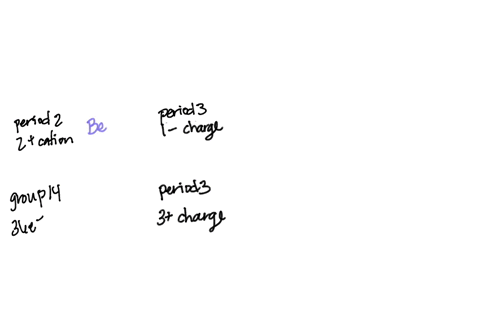
SOLVED: Question 24 1 pts An atom of an element forms stable ion by easily losing electrons. The ion has charge 2 and is in Group 2 2 and is in Group
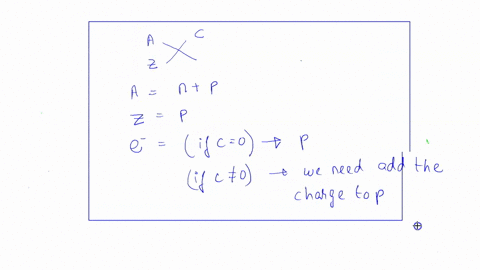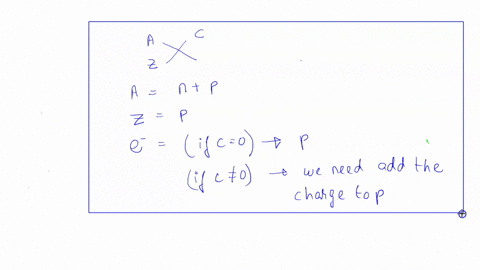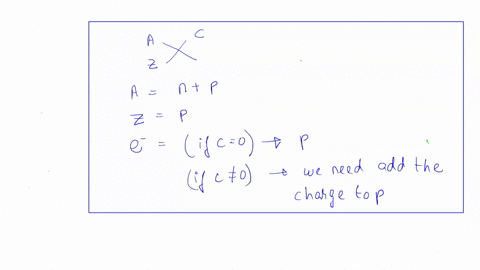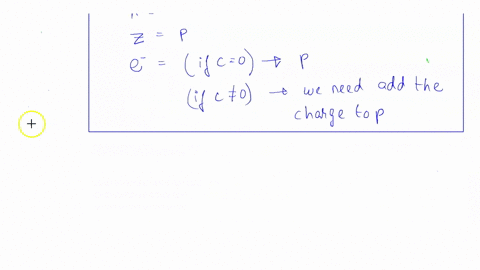
SOLVED: 'Activity 2. Negative Ions (Anions) Direction: Determine the number of electron, proton and neutron in negative ions (anion): Write your answer inside the box on the space provided: 35 17 Cl
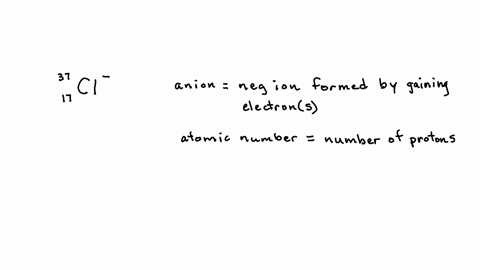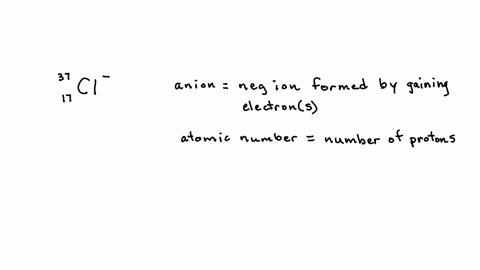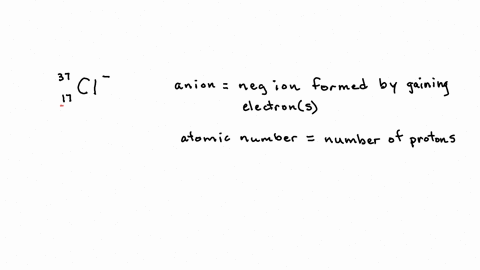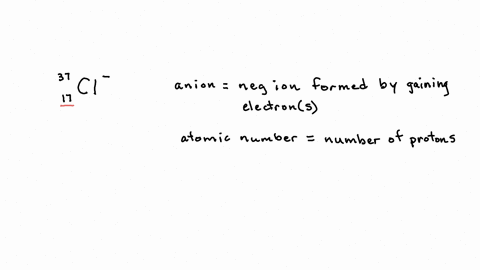
SOLVED: 'Activity 2. Negative Ions (Anions) Direction: Determine the number of electron, proton and neutron in negative ions (anion): Write your answer inside the box on the space provided: 35 17 Cl
Atomic Structure d. Atomic Structure d Atomic Structure d Electron (negative) Neutron (neutral) Proton (positive) d nucleus. - ppt download

Chapter 5, Nomenclature Video Solutions, Introductory Chemistry
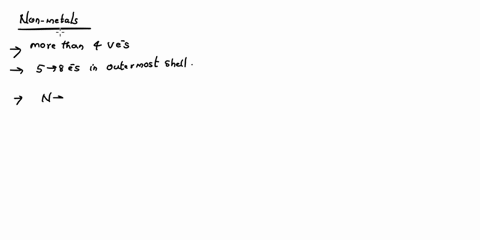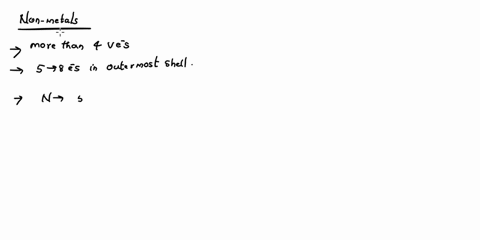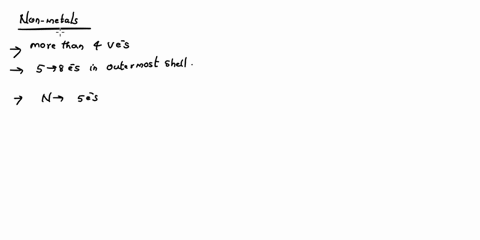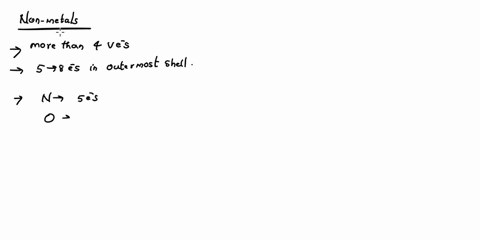
SOLVED: in many compounds, atoms of main-group elements form ions so that the number in the outermost energy levels of each ion is
- WHAT ARE NEGATIVE IONS?
- Why is a positive ion often surrounded by eight negative ions? - Quora
- For each of the negative ions listed in column 1, use the periodic

- Bad News: Those Popular Negative Ion Bands Are Secretly Sending
- Forming negative and positive ions - Bonding - (CCEA) - GCSE Chemistry (Single Science) Revision - CCEA - BBC Bitesize
- Y-3 Cuffed Jogger - Black - IV5570

- Calvin Klein Performance Womens Plus Fitness Workout Athletic Leggings Black 3X

- La Belle Époque / an essay by Philippe Jullian ; with

- 1 Pcs Mercase Posture Corrector for Men and Women, Back Brace for Posture, Adjustable and Comfortable, Pain Relief for Back,Shoulders,Neck,Nude S-M

- Satin and Lace Bridal and Bridesmaid Robes