42g of N₂ react with excess of O₂ to produce NO. Amount of NO formed is a.60g b.32g c.45g d.90g

By A Mystery Man Writer
Share your videos with friends, family and the world

Empirical Formula from Combustion - Carbon, Hydrogen AND oxygen

Limiting Reagent What happens in a chemical reaction, if there is

WO2022045231A1 - Ester compound - Google Patents

Answered: if 24.0g of NO and 13.8g of O2 are used…

Consider the reaction: NO2( g) ¡ NO( g) + 1 2 O2( g) The tabulate

Topical Mock Chemistry Questions, PDF
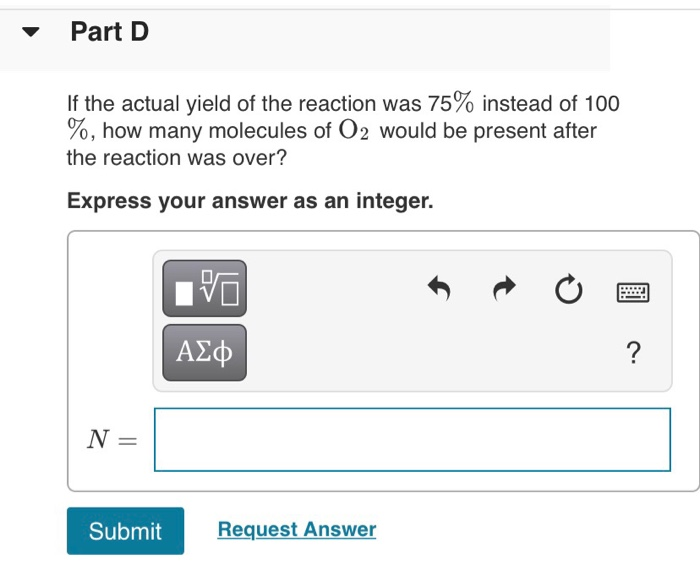
Solved Nitrogen monoxide and oxygen react to form nitrogen

JP2022531876A - Convergent liquid phase synthesis of

5.2: Reaction Stoichiometry (Problems) - Chemistry LibreTexts
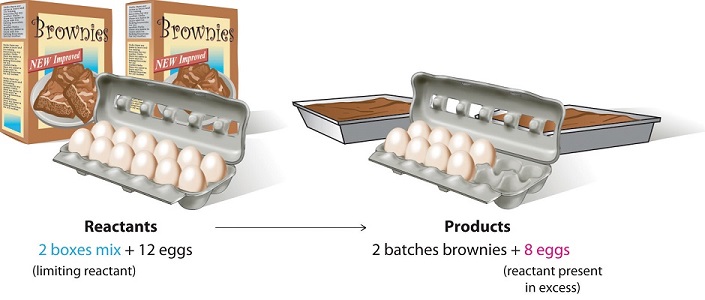
4.3: Limiting Reactant, Theoretical Yield, and Percent Yield

Solutions Dinesh, PDF, Molar Concentration
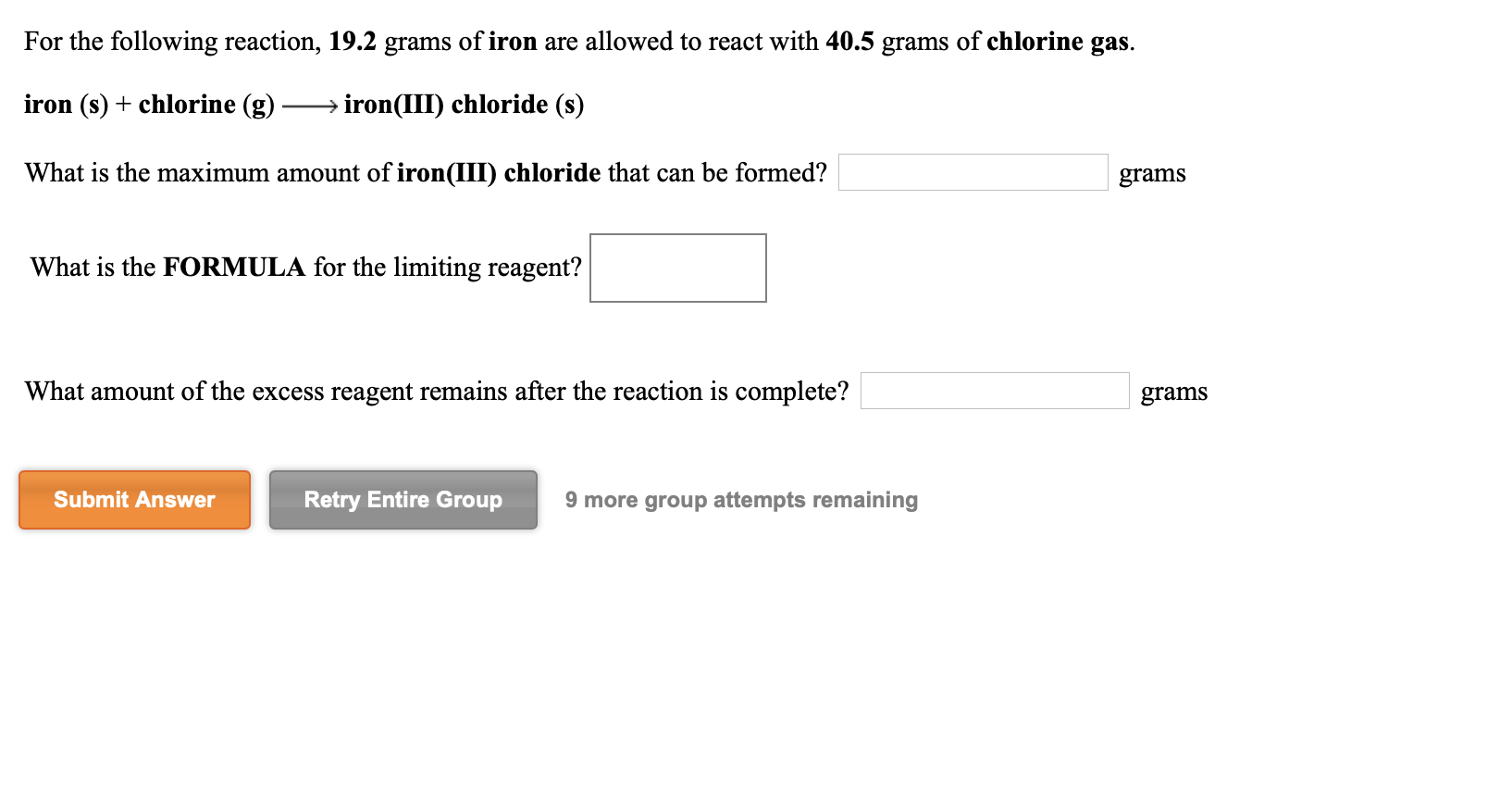
Solved For the following reaction, 8.79 grams of nitrogen

UMAIR KHAN ACADEMY

Solutions Dinesh, PDF, Molar Concentration
- 18.5 to 29cm 4 size choice Toilet tank fittings kit Dual-flush toilet repair kit Suitable

- Ropa De Gimnasio Para Hombre, Conjunto De Compresión, Traje De Entrenamiento Para Trotar, Ropa Deportiva Para Hombre, Mallas Deportivas Para Correr, Camisa De Entrenamiento + Pantalones - Sets - AliExpress

- PINK by VS bralette. Salmon/Peach color. Worn once - Depop

- Women Yellow Lemon High Waisted Bikini Set Two Piece Swimsuits Push Up Wrap Swim Suits Top Tummy Control Bathing Suit Bottom S

- Azar Displays ACRYLIC LOCKBOX COUNTERTOP DISPLAY CASE 255400 - The Home Depot





