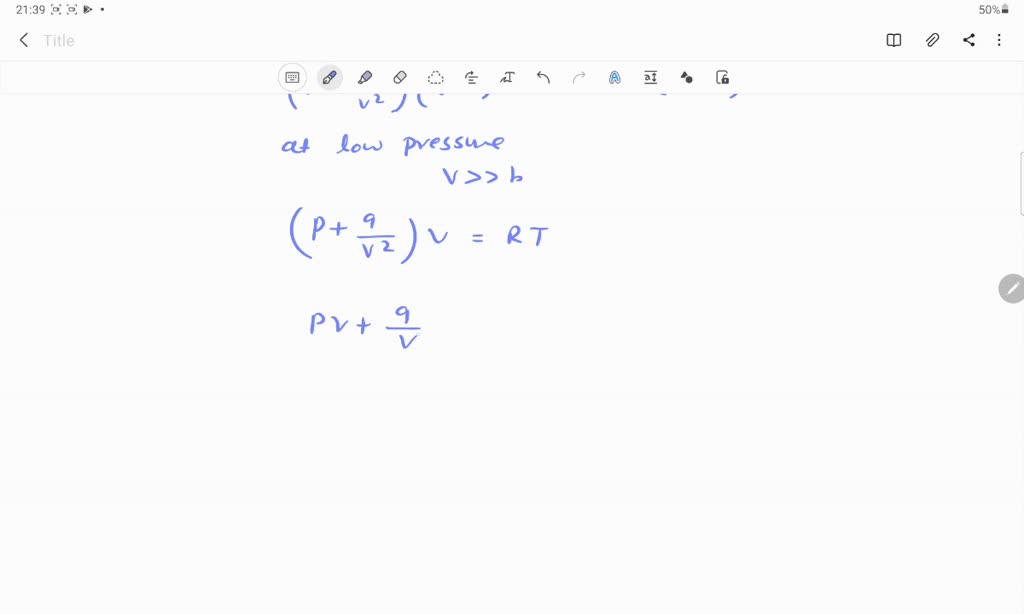
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to
By A Mystery Man Writer
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0

At low pressure, the van der Waals equation is reduced to

At low pressure, the vander Waal's equation >re become : (1) PV

At low pressure, the van der Waals equation is reduced to
⏩SOLVED:If Z is a compressibility factor, van der Waals equation

Van der Waals equation, when pressure correction is ignored, one

physical chemistry - Why do some gases have lower value of Z for a

Solved 2. (20 points) At low pressures, the compressibility

JEE: Van der Waals Equation, Chemistry By Unacademy

2. U 0.52, 0.68, 0.74 At low pressure, the comprensibility factor
- Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor

- Compressibility Factor Calculator

- Thermodynamics - 3-7 Ideal Gas Equation with compressibility factor example 1

- If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

- Compressibility Factor Z Important Concepts and Tips for JEE Main

- Women's Activewear - Shorts & Joggers - TRM Clothing Ireland

- Movie HiGH & LOW THE WORST Sequel (tentative) to be screened in

- BMC Bruidsmeisjes op Instagram: NEW COLLECTION VENLO ~ Caftan black ~ Size 38/42 ~ Rentalprice/huurprijs; €50 #moroccandress #venlo #rental…

- Lilac Period Underwear at Rs 499/piece

- NWT Z by Zobha The Outsider Active Women Legging Running Jog Gym Tight Pants $75
