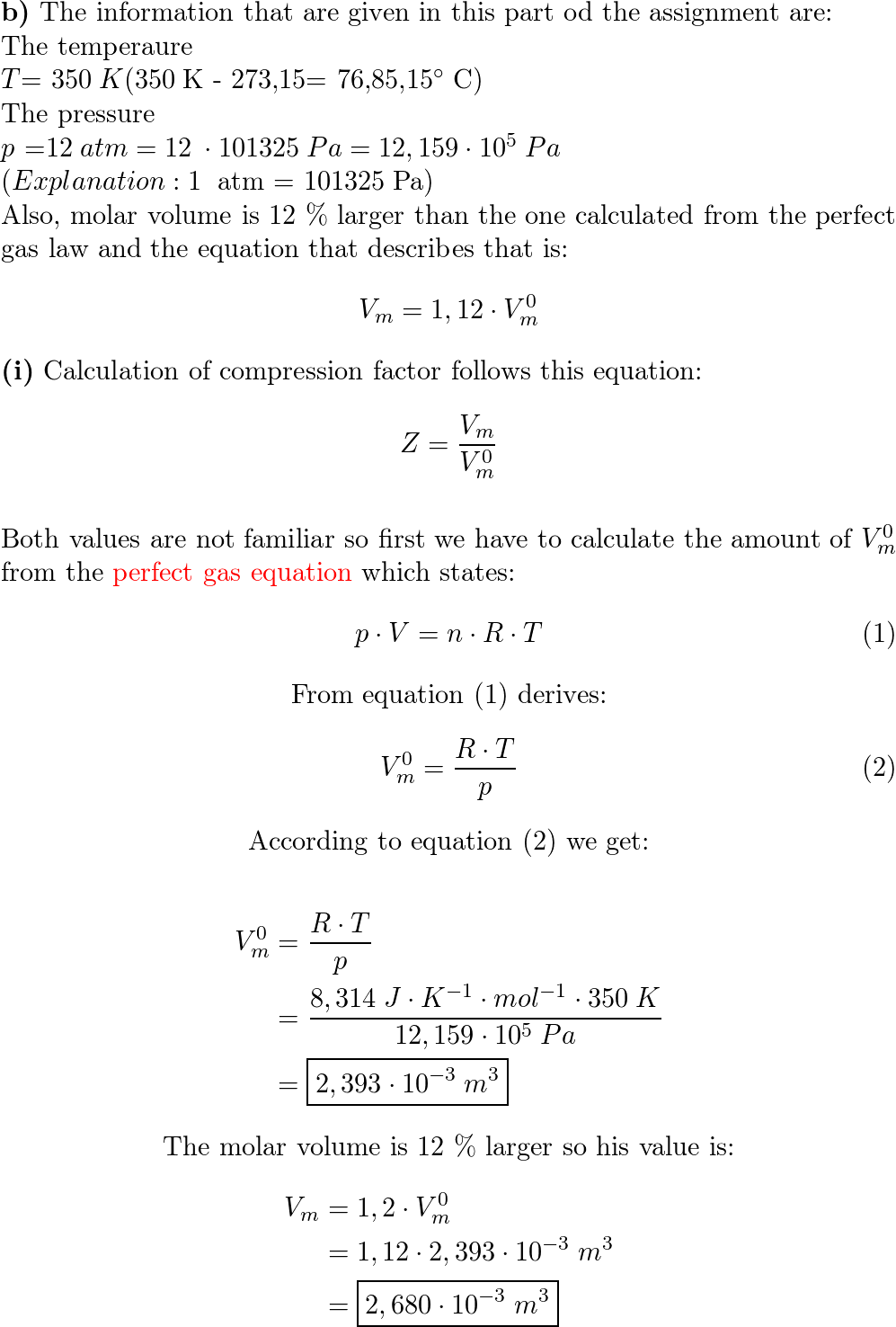
Sunday, Sept 29 2024
Solved] Why is the compressibility factor less than 1 at most
By A Mystery Man Writer
Answer to Why is the compressibility factor less than 1 at most conditions?

SOLVED: Problem 1: Answer the following concept-related questions

3.2 Real gas and compressibility factor – Introduction to

Compressibility Factor of Gas Overview, Equation & Chart

Compressibility Factor - an overview

The compressibility factor (Z) of real gas is usually less than 1 at l

States of Matter Class 11 Notes CBSE Chemistry Chapter 5 [PDF]
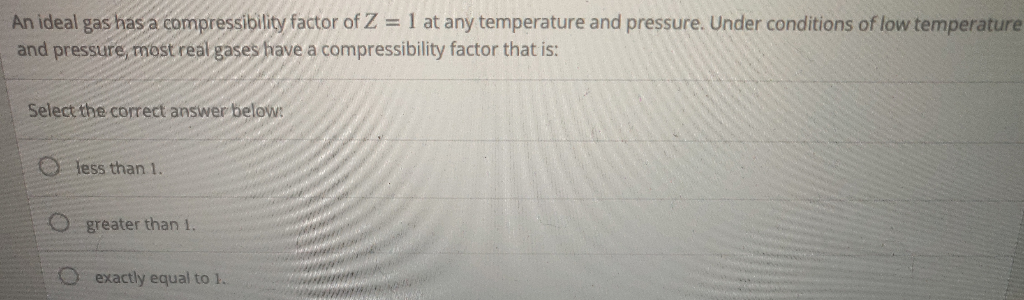
Solved An ideal gas has a compressibility factor of Z = 1 at
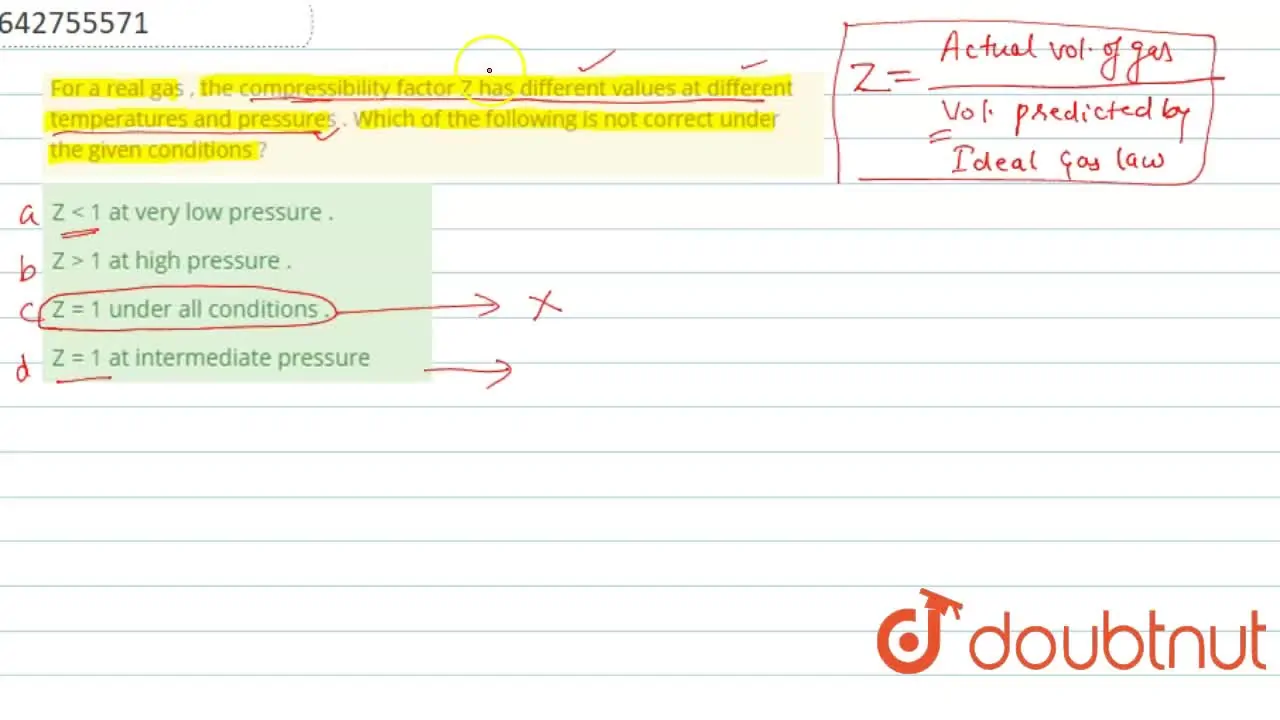
For a real gas , the compressibility factor Z has different values at

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

Compressibility factor - Wikipedia
Related searches
Related searches
©2016-2024, doctommy.com, Inc. or its affiliates