FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice

By A Mystery Man Writer
The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

Hundreds Of Thousands Of Medtronic Insulin Pumps Recalled

Medtronic MiniMed Insulin Pump Lawsuit

Health Canada licenses Medtronic infusion set for insulin delivery

Medtronic expands Class I recall of MiniMed 600 series insulin
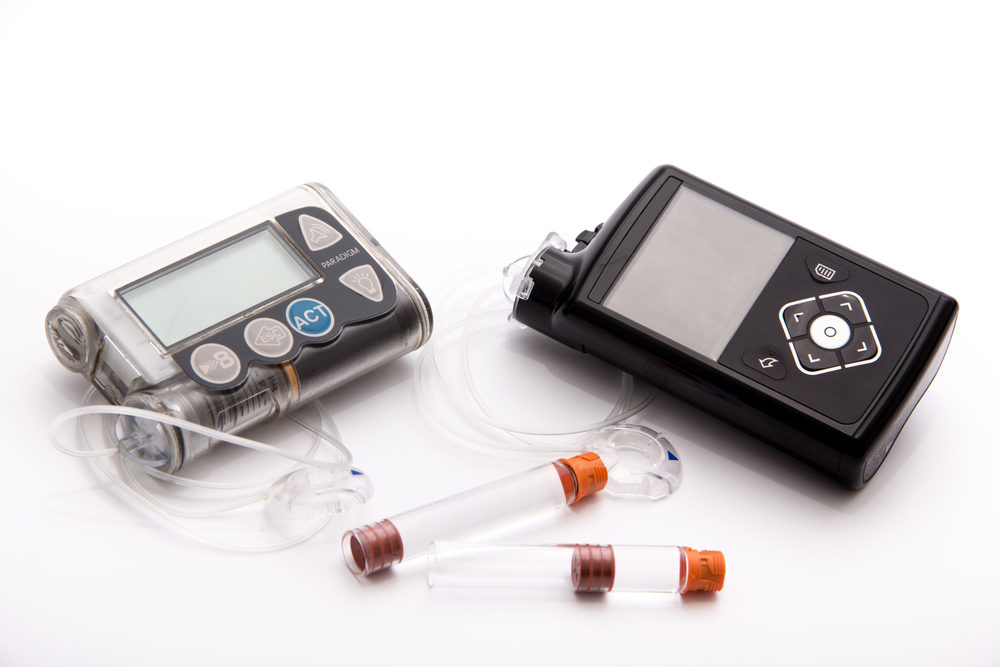
Medtronic Recalls More MiniMed Insulin Pumps for Dosage Issues
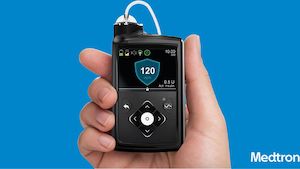
Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Medtronic MiniMed Insulin Pump Lawsuit

Food & Drug Administration (FDA) Archives - Page 5 of 84 - Drug Delivery Business

Medtronic - Wikipedia

Medtronic MiniMed Insulin Pump Lawsuit March 2024 - Select Justice

Report: Literal killer app prompted Medtronic MiniMed recall - Medical Design and Outsourcing

MiniMed 780G System – P160017/S091

Medtronic CEO confirms FDA warning could affect approval timing
- Insulin Pump - Health Library
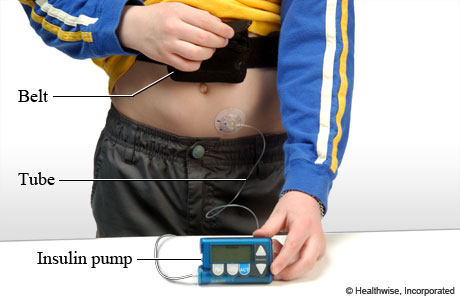
- 2.1 Pumps with and without tubing
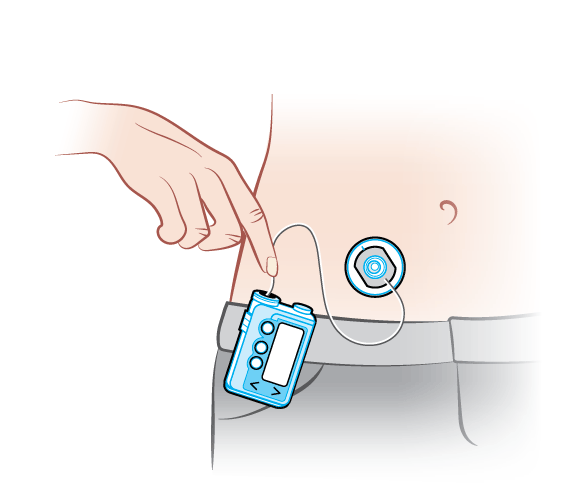
- Insulin Patch Pump System - Shore

- Insulin pumps

- DiaBelt Insulin Pump Belt with Mesh Pouch for Easy Viewing Operation, Diabetic T1D Medical Holder Accessories Waist Band with Slits for Tubing Epipen Men Women Adult Black : Health & Household





