The vapour pressure of a solution having 2.0 g of solute X (gram
By A Mystery Man Writer
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
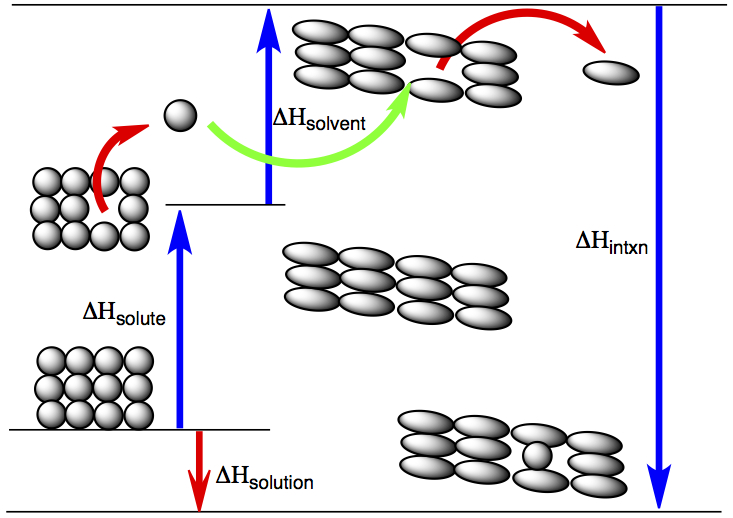
Chapter 12 Solutions and Their Properties

In ideal solution of non volatile solute B in solvent A in 2 : 5 molar ratio has vapour pressure 250

my lioperties, Abnormality in Molar Mass) 2. The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol-') in 100 g vapour pressure =

Molar Mass from Osmotic Pressure
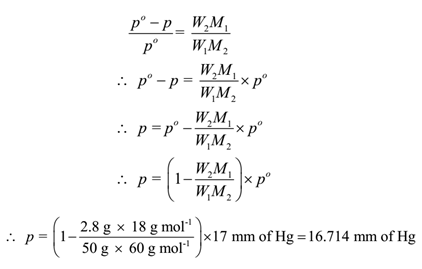
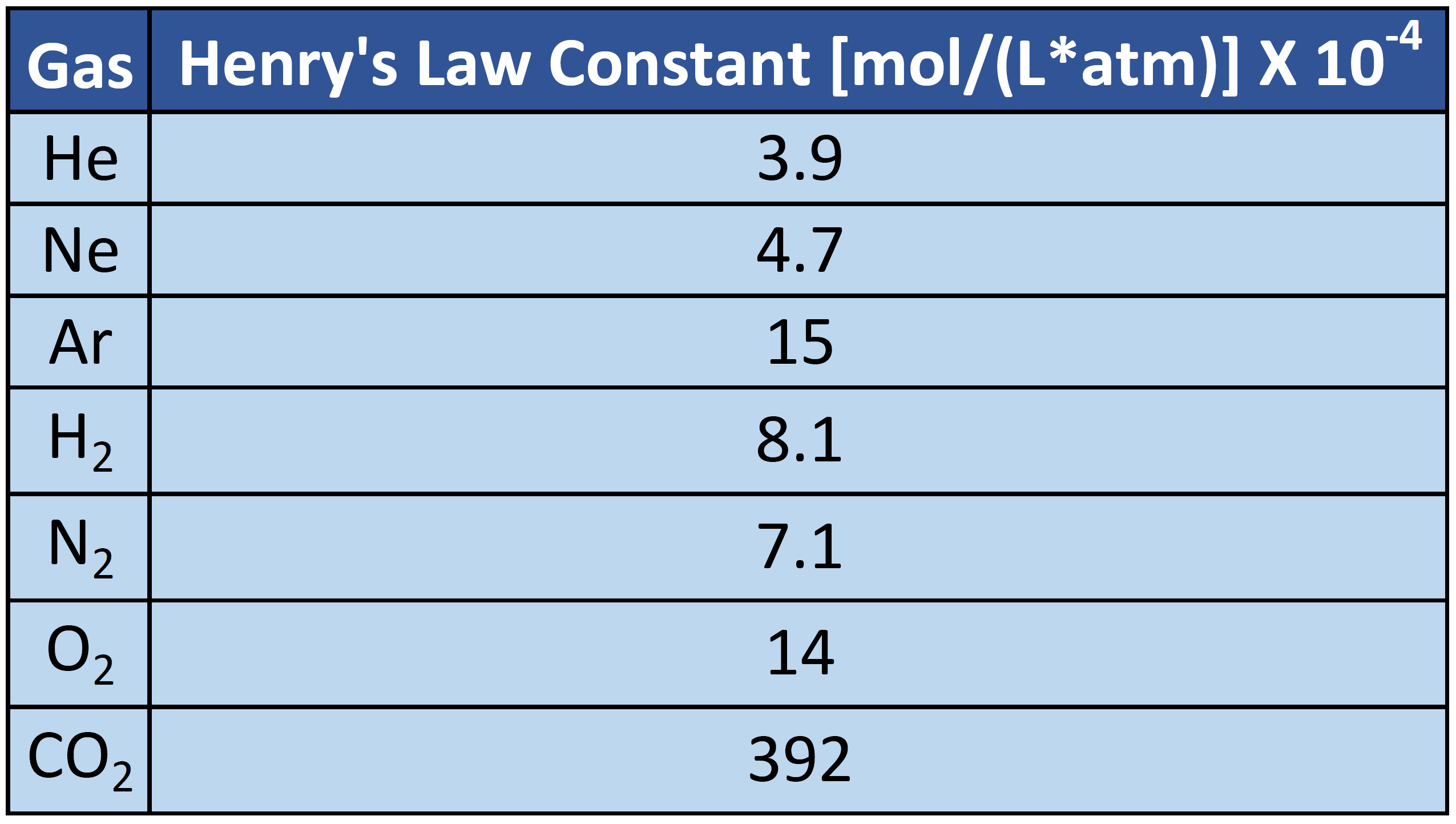
Lowering of Vapour Pressure: Numerical problems with solutions
CH104: Chapter 7 - Solutions - Chemistry
Lec 7. Solutions and Colligative Properties - TIB AV-Portal

Solved 1. Assuming Raoult's Law applies, calculate the vapor

Answered: Calculate the vapor pressure at 35…
- Tempero em Pó KNORR Carne 32 g 8 sachês

- Yakitori Seasoning Mix 32g, Search, Products

- HALLS Rebuçados Morango Sem Açúcar 32 g, CARAMELOS DUROS

- Tablet M10A 3G Android 9 Pie 32 Gb Dual Câmera 10 Polegadas Quad Core Preto Nb331 Multilaser - Tablet Multilaser - Magazine Luiza

- Tablet SPC Gravity 3 SE (10.35'' - 32 GB - 2 GB RAM - Cinzento
- Understanding Social Security Benefits - Ramsey

- Young Woman With Big Breasts Sits On A Table In An Office Near A Laptop. Stock Photo, Picture and Royalty Free Image. Image 136906566.

- Fresh Keeping Bag, 100pcs Elastic Stretchable Food Storage Covers,Kitchen Universal Packaging Seal Fresh Food Covers, Food Preservation Covers For

- Junior Girls' [7-16] Crossback Sports Bra, Under Armour
- Simply Vera Vera Wang Women's Brown Luxe Cotton Leggings (VW5226

