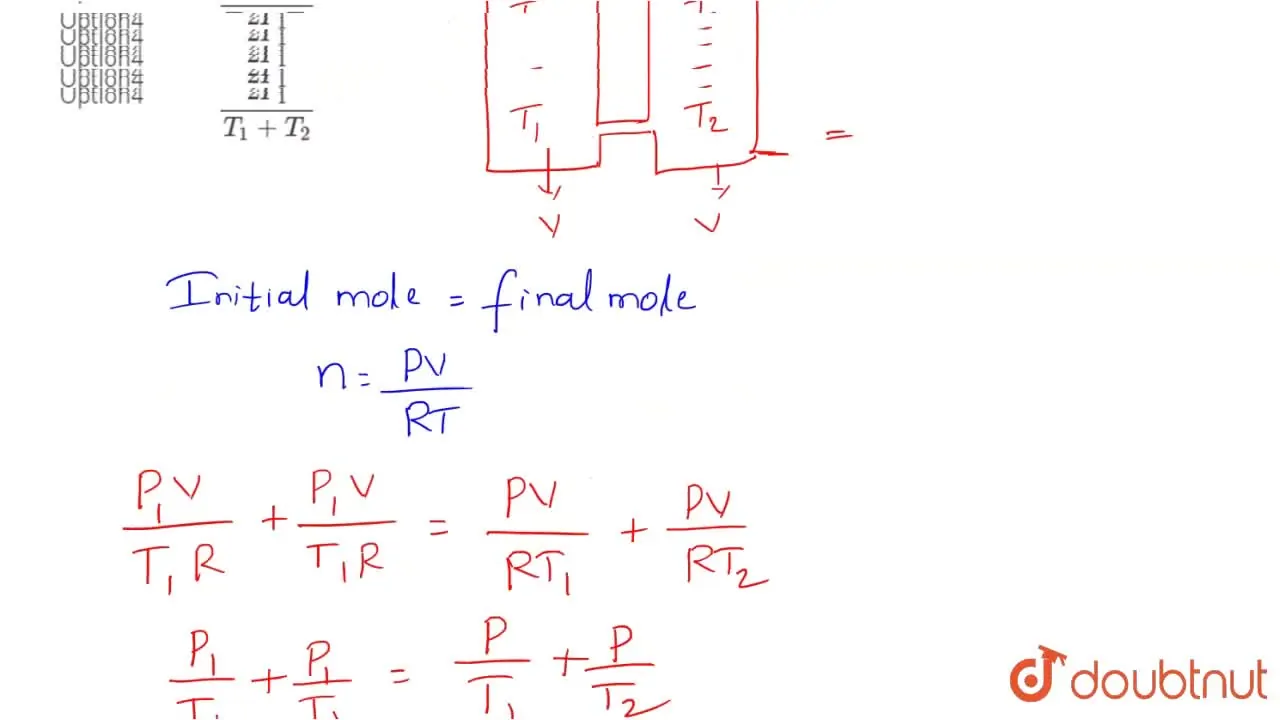
Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in

By A Mystery Man Writer
Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T 2. The final pressure Pf is :P i T 1 T 2/ T 1+ T 2B. 2 P i T 1/ T 1+ T 2C. 2 P i T 1 T 2/ T 1+ T 2D. 2 P i T 2/ T 1+ T 2
Two closed bulbs of equal volume V containing an ideal gas initially at pressure P i and temperature T 1 are connected through a narrow tube of negligible volume as shown in the figure below- The temperature of one of the bulbs is then raised to T 2- The final pressure Pf is -P i T 1 T 2- T 1- T 2B- 2 P i T 1- T 1- T 2C- 2 P i T 1 T 2- T 1- T 2D- 2 P i T 2- T 1- T 2
The correct option is D 2P_i ( T_2T_1+T_2 )Since the above system is a closed one, the total number of moles of the ideal gas will be equal before and after th
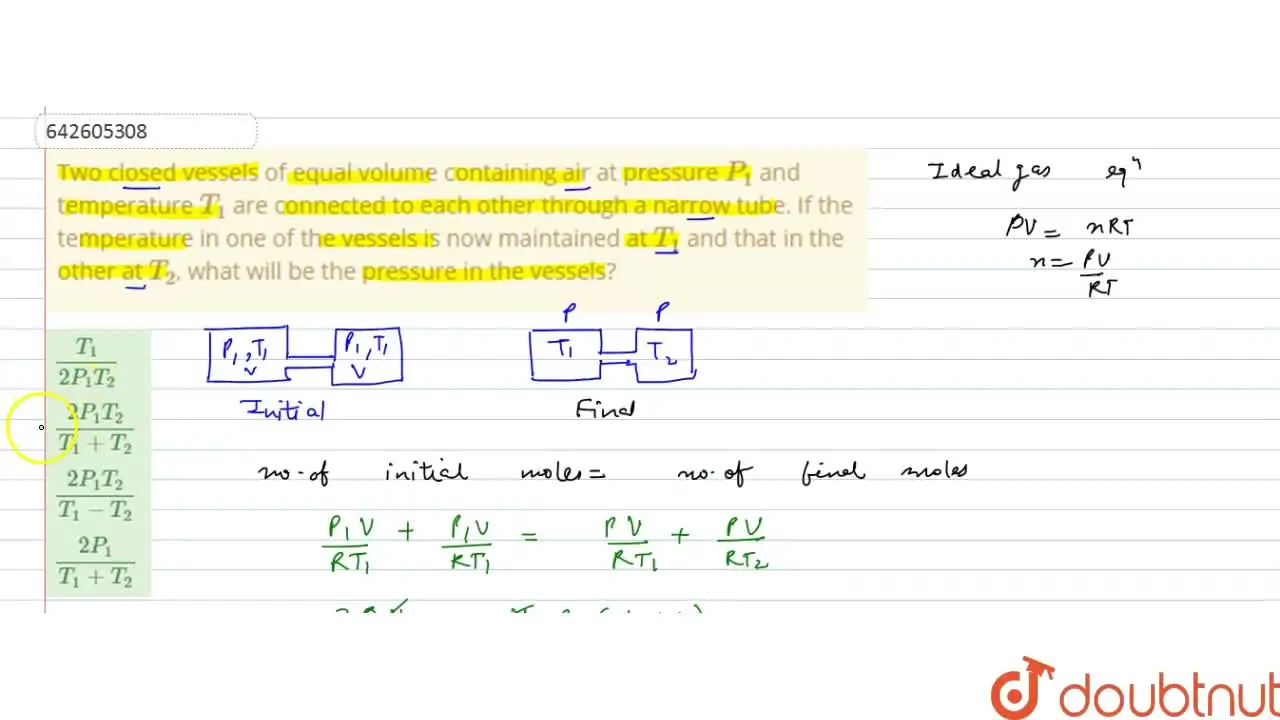
Two closed vessels of equal volume containing air at pressure P(1) and

Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure pi and temperature T1 are connected - Chemistry - Solutions - 12339331
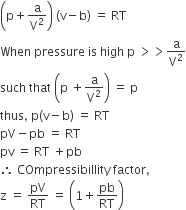
The compressibility factor for a real gas at high pressure is f
Bengali] Two bulbs of equal volume are connected by a narrow tube of

Nisha Kumari - Student - IIT KANPUR - EduRev HAJIPUR BIHAR

Two closed bulbs of equal volume (V) containing an ideal gas initially at pressure p_i and temper


Gaseous State - 2 Free MCQ Practice Test with Solutions - Chemistry
Telugu] Two closed vessel A and B of equal volume containing air at p

Two closed bulbs of equal volume (V) containing an ideal gas initially pressure p and temperature T. are connected through a narrow tube of negligible volume as shown in the figure below.
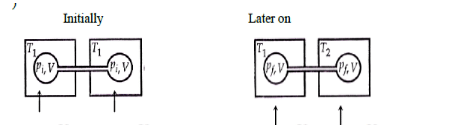
Two closed bulbs of equal volume $(V)$ containing

⏩SOLVED:Two closed bulbs of equal volume ( V ) containing an ideal…
- Fonte 5V 3A Micro Usb Ideal para Raspberry Pi - AutoCore Robótica - Arduino em Fortaleza, você encontra aqui!

- Givenchy Pi 3.3 oz EDT Spray mens cologne 100 ml NIB 3274878222568

- Ideal Deepness I Canvas Print by PI Studio
- Ideal Deepness II Art: Canvas Prints, Frames & Posters

- SOLVED: ideal gas initially at Pi, Vi, and Ti is taken through
- Breg TScope Premier Post Op Knee BraceCool

- Womens Comfort Minimizer Bra Wirefree Non Padded Seamless Full Coverage Bra Plus

- Camiseta Dry Fit Masculina 100% Poliester Academia Corrida - Tok

- Hero Biker Leather Jacket – Wings Of Liberty Clothing

- CRZ YOGA Women's Compression Workout Leggings 25/28 The Cognac Brown XX-Small : Clothing, Shoes & Jewelry
