physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

By A Mystery Man Writer
In the above graph,the minima of the curve for methane is more than that of nitrogen. Also, for a given value of pressure, the value of $Z$ for methane is less than that of nitrogen. They seem to m
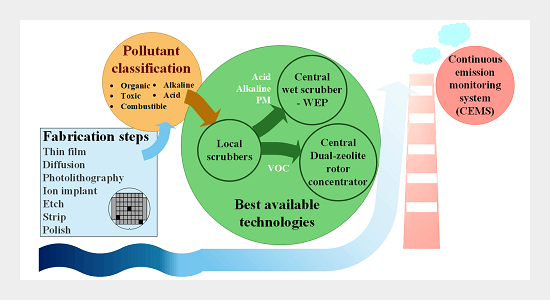
Continuous Improvements and Future Challenges of Air Pollution
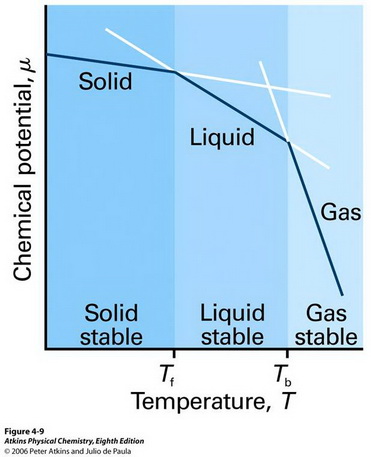
How do the chemical potentials compare for the vapour and liquid
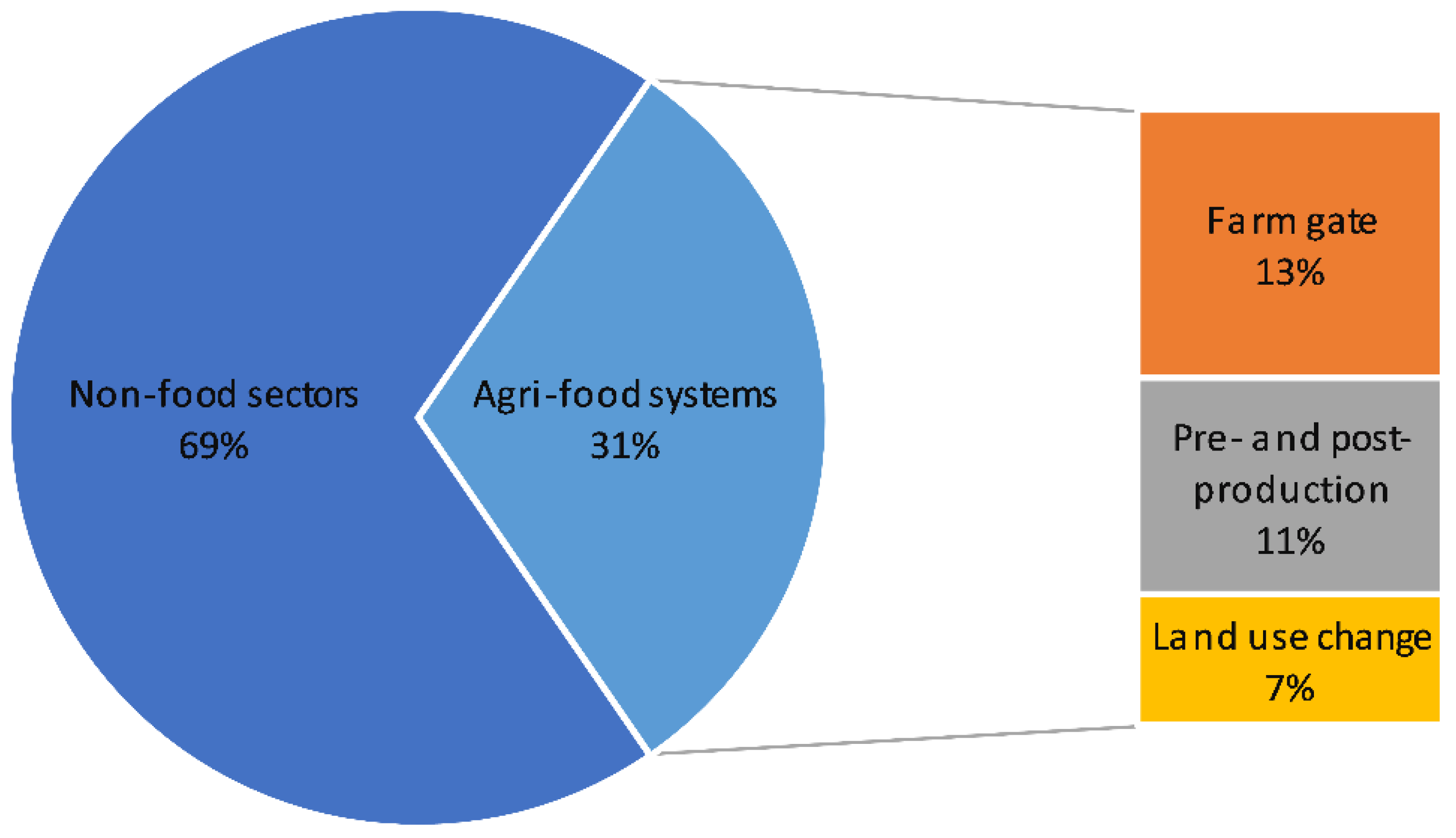
Agriculture, Free Full-Text

Chemistry - Unit 3 - Joseph Flashcards

Minerals, Free Full-Text
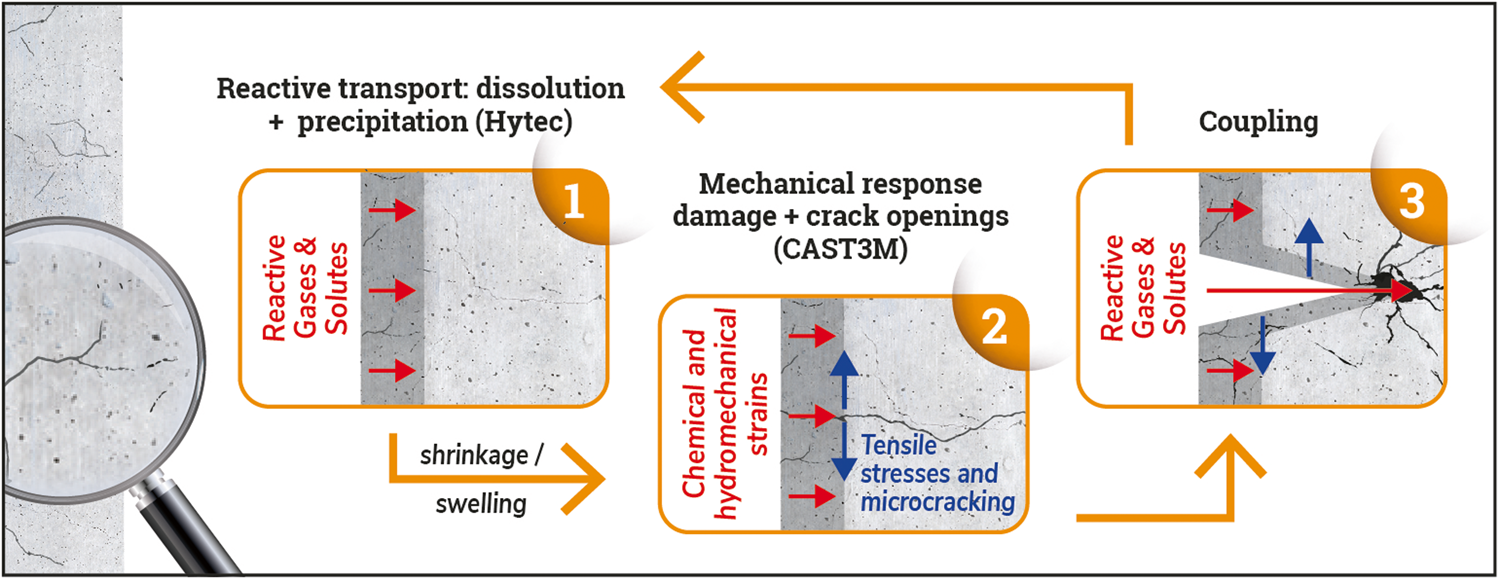
A fully coupled Hydraulic Mechanical Chemical approach applied to

Gas - Wikipedia
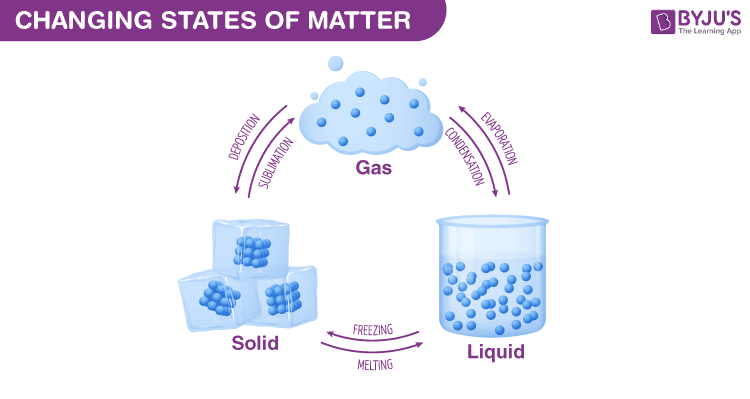
Changing States Of Matter - Solid, Liquid And Gas
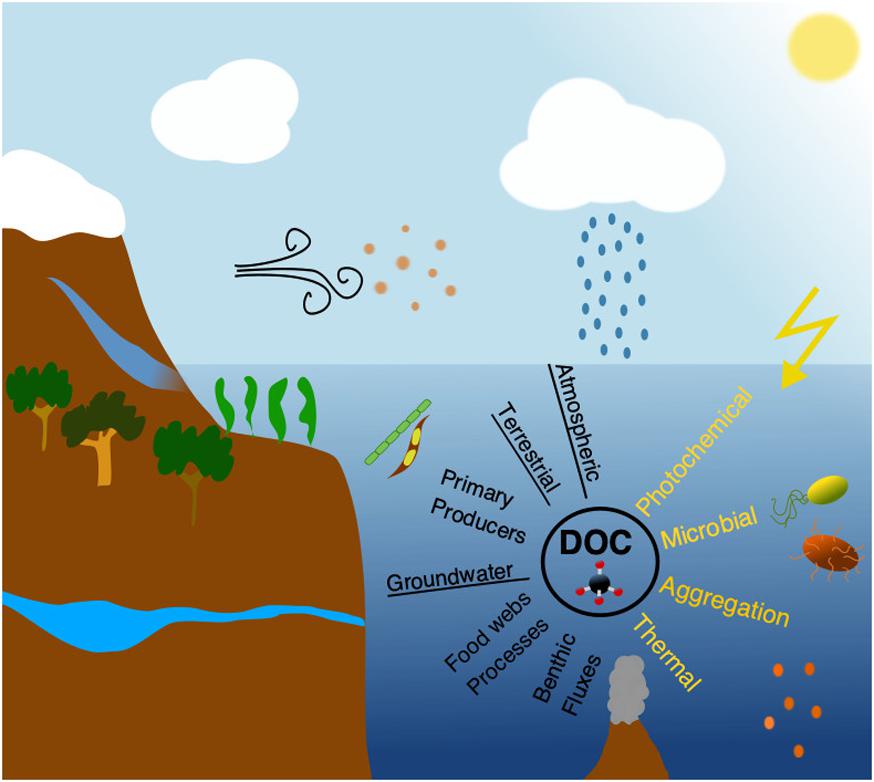
Frontiers Impacts of Global Change on Ocean Dissolved Organic
What is the significance of the curve part in Z vs. P graph of
CH103 - CHAPTER 2: Atoms and the Periodic Table - Chemistry
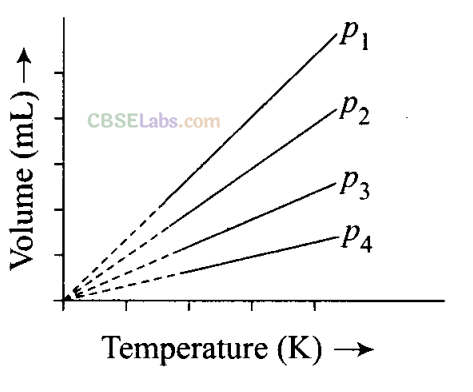
NCERT Exemplar Class 11 Chemistry Chapter 5 States of Matter
Why would it be impossible for the pressure of the gas to be
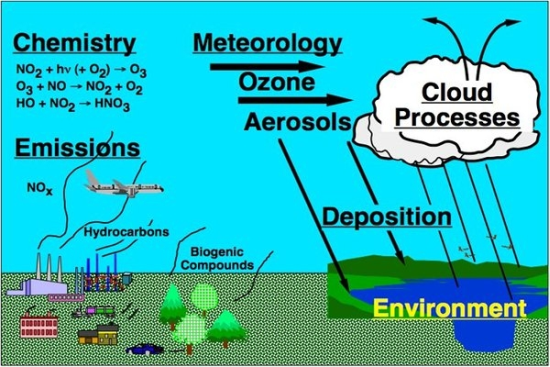
Atmosphere, Free Full-Text
- Explain how the compression factor varies with pressure and

- Answered: (a)Using the compressibility chart,…

- What is compressibility factor? What is its value for ideal gas

- Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
- Plot of experimental measurements of the z-factor

- Columbia ENDLESS TRAIL™ RUNNING 7/8 - Leggings - black

- Women's Bras Pink Soda Sports Bra Lingerie

- Portion Scoop - #16 (2 oz) - Disher, Cookie Scoop, Food Scoop - Portion Control - 18/8 Stainless Steel, Blue Handle

- Bally, Pants & Jumpsuits, New Bally Total Fitness Tummy Control Legging Size Small

- Gymshark, Tops

