organic chemistry - Why is this diagram depicting the molecular orbital (MO) basis for a back-side attack the way it is? - Chemistry Stack Exchange

By A Mystery Man Writer
Consider: The description of this image in my textbook is as follows: In order to form a bond, the HOMO (the highest occupied molecular orbital) of one species must interact with the LUMO (the lo

organic chemistry - When is donation into an anti-bonding MO stabilising? - Chemistry Stack Exchange

Present State of the Art and Future Challenges in the Hydrodesulfurization of Polyaromatic Sulfur Compounds - ScienceDirect
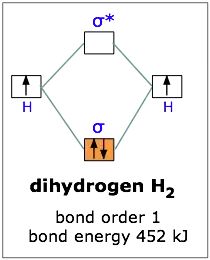
Molecular orbital theory & predicting the stability of a molecule? - Chemistry Stack Exchange
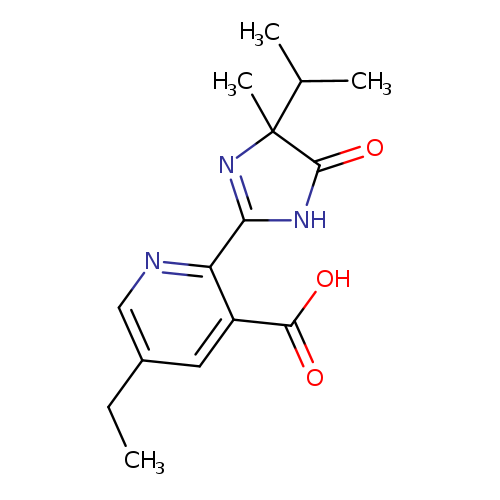
81335-77-5, MFCD00274561, Imazethapyr

The CO molecule is an important ligand in Inorganic chemistry. Generate a molecular orbital diagram for this molecule. For purposes of simplification you may consider only the 2p set on the oxygen

In Molecular Orbital Theory, why do sigma and sigma* bonding and antibonding orbitals have a greater difference of energy than pi and pi* orbitals? - Quora
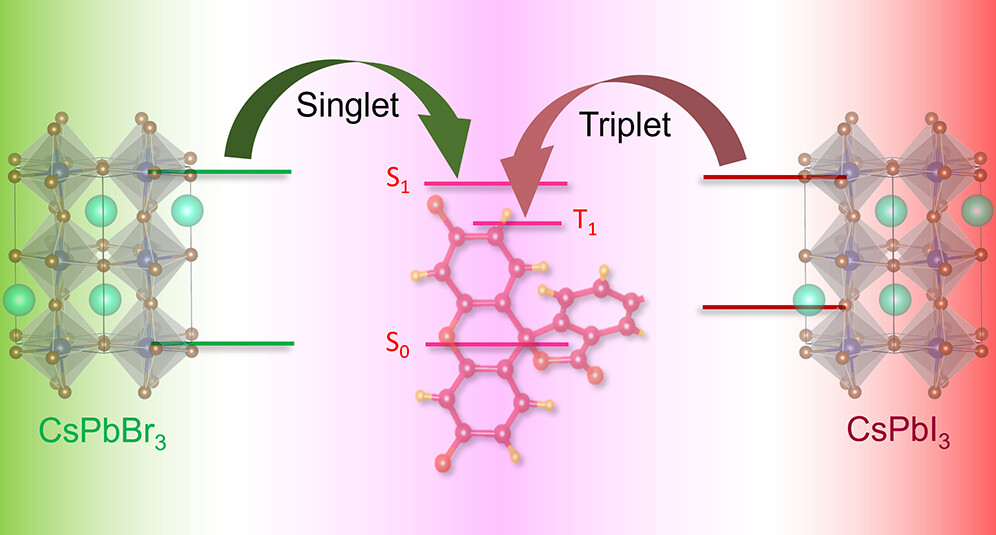
All Publications
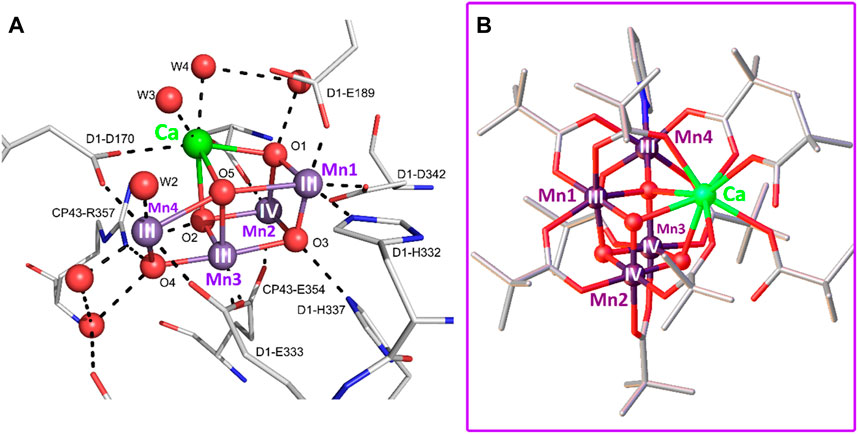
Frontiers Bioinspired polyoxometalates as light-driven water oxidation catalysts

Oxygen reduction electrochemistry at F doped carbons: A review on the effect of highly polarized C-F bonding in catalysis and stability of fuel cell catalysts - ScienceDirect

1.4 Molecular Orbital Theory

Chemistry and Lithography, Second Edition, Vol. 2: Chemistry in Lithography [2 ed.] 1510655573, 9781510655577
- APC Back-UPS Pro BR - UPS - AC 120 V - 600 Watt - 1000 VA - USB, serial - output connectors: 10 - black

- Quem é Neuza Back, árbitra brasileira que fará parte do 1º trio

- Richarlison gets new back tattoo of Neymar, Ronaldo and himself - Futbol on FanNation

- I Got My Joy Back. Nba pictures, Football quotes, Sports quotes

- Back injury takes Bia Haddad out of Wimbledon tournament

- Dos and Don'ts of Finding Love at the Gym - Fit Athletic – San Diego Best Gym

- Voices of Syrian women in civil resistance

- Nike Performance BRASIL CBF TRAVEL HOODIE - Zip-up sweatshirt - coastal blue/green spark/dynamic yellow/blue

- Niidor Sticky bras on LinkedIn: Niidor adhesive bra is made of
- Terra & Sky Women's Plus Size Floral Printed Leggings

