32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
By A Mystery Man Writer
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

Chapter 3.powerpoint
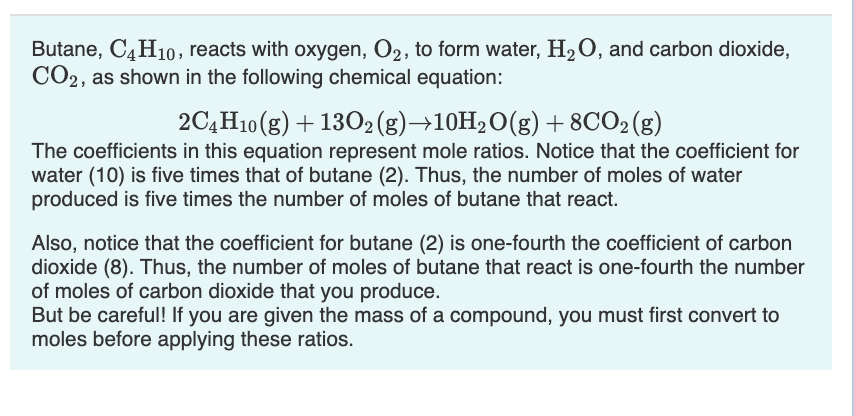
Solved Butane, C4H10, reacts with oxygen, O2, to form water
How much mass of water is obtained by reacting 80 g each of hydrogen and oxygen? - Quora
Catalysts, Free Full-Text

80 gram of H2 is reacted with 80 gram of O2 to form water find out the mass of water obtained which

Spatiotemporal Decoupling of Water Electrolysis for Dual-Use Grid Energy Storage and Hydrogen Generation - ScienceDirect

Q. 88.6 mathrm{g} of mathrm{H}_{2} reacts with 32 mathrm{g} of mathrm{O}_{2} to yield water. Which is the limiting reactant? Find the mass of water produced and the amount of excess reagent left.
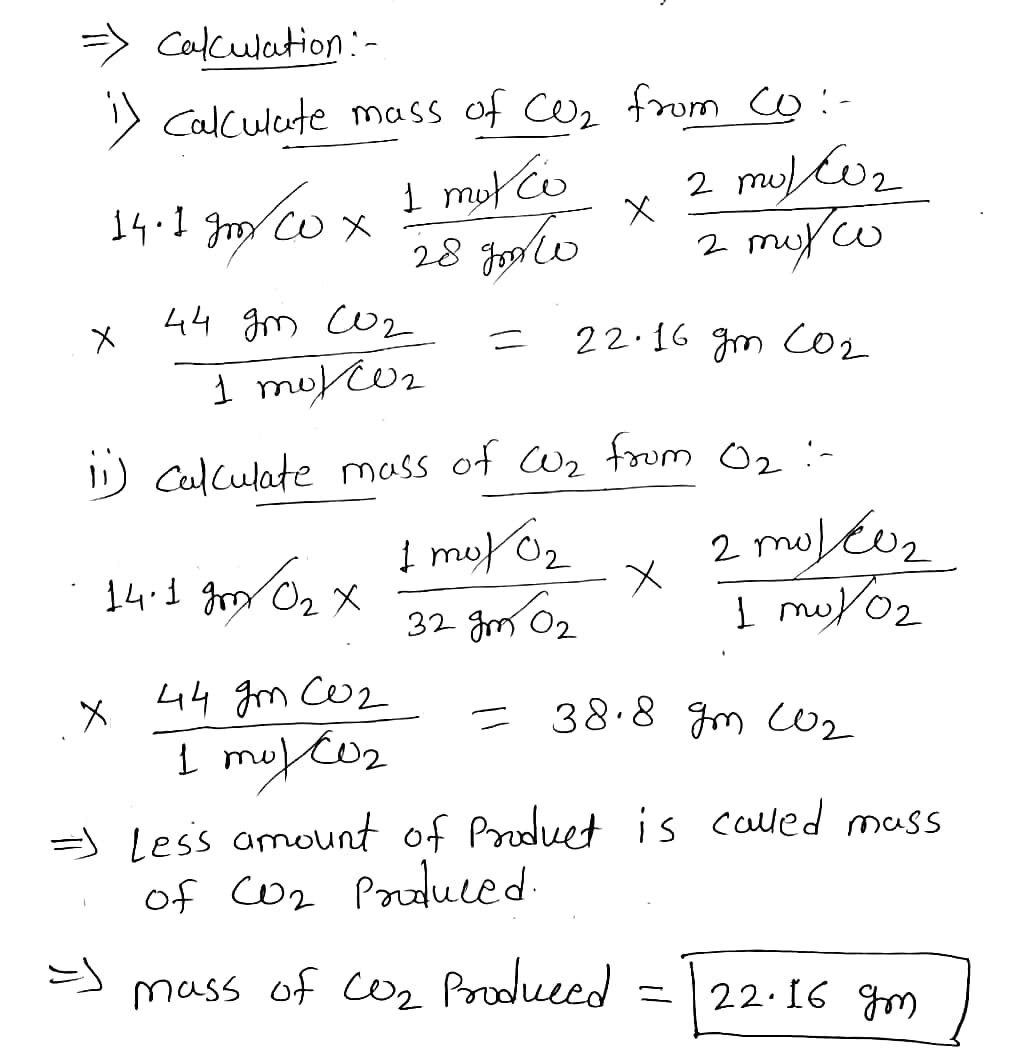
Answered: A reaction vessel contains14.1 g of CO…

80 g of h2 is reacted - Chemistry - Chemical Kinetics - 14366697

Question Video: Calculating the Mass of Water Produced Given the Masses of Oxygen and Hydrogen

Materials, Free Full-Text

US10328082B2 - Methods of use and combinations - Google Patents

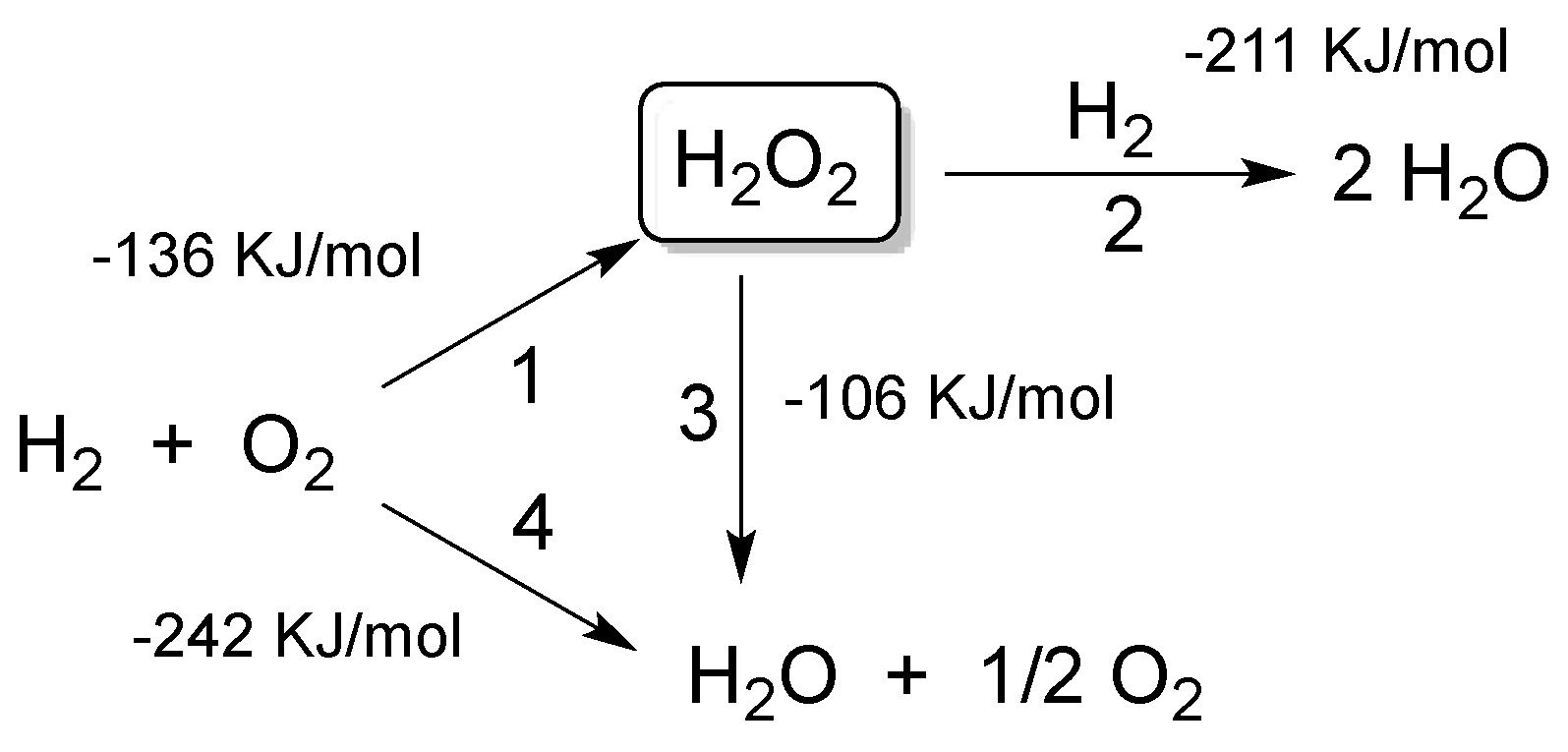
Direct production of H2O2 from H2 and O2 in a biphasic H2O/scCO2 system over a Pd/C catalyst: Optimization of reaction conditions - ScienceDirect

Solved Question 2 • How many grams of water are produced if

Malayalam] Find out the limiting reagent when 5g of H2 reacts with 24
- armani exchange underwear products for sale

- Women Sheer Mesh Leggings Pants See Through Low Waist Booty Slim Skinny Sexy

- Shapewear For Tummy Control Faja Plus Size Butt Lifter Body Shaper

- Surge mais um nome possível para ser vice de Moacyr de Almeida em Sidrolândia - Four News

- Black Lace Tops for Women Elegant Long Sleeve Sexy Button V Neck