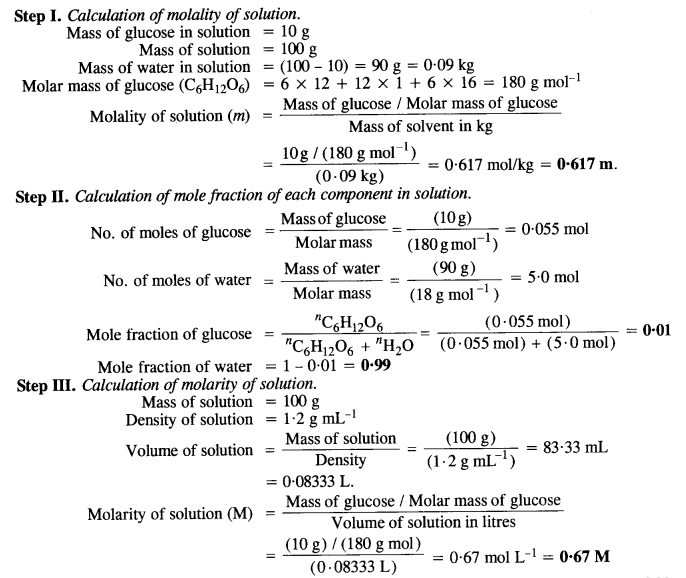
2.t 300 K, 36 g of glucose present per litre in itssolution has an
By A Mystery Man Writer
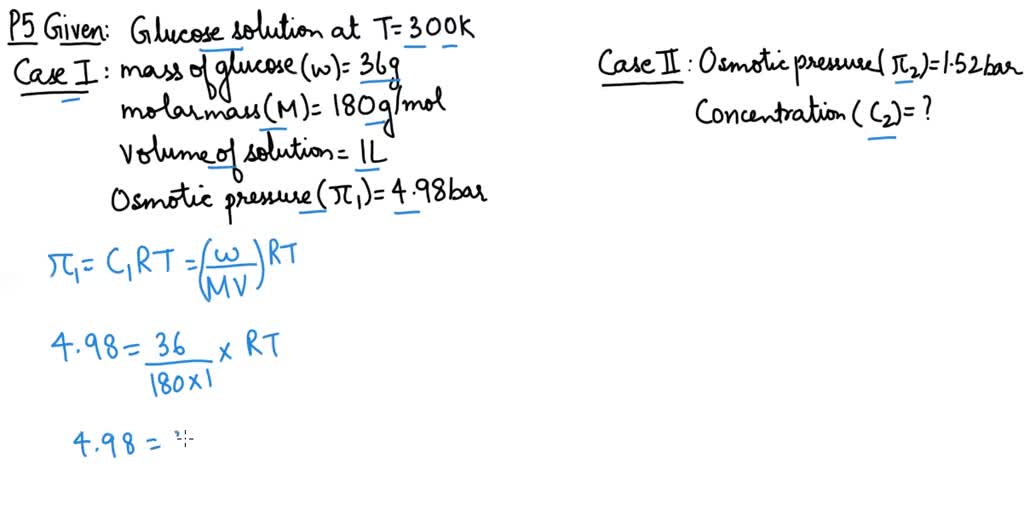
2.t 300 K, 36 g of glucose present per litre in itssolution has an osmotic pressure of 4.98 bar. If theosmotic pressure of solution is 1.52 bar at thesame temperature, what would be itsconcentration?(1) 11 gl 1(3) 36 gl 1(2) 22 gL 1(4) 42 gL 1
2-t 300 K- 36 g of glucose present per litre in itssolution has an osmotic pressure of 4-98 bar- If theosmotic pressure of solution is 1-52 bar at thesame temperature- what would be itsconcentration-1- 11 gl-1-3- 36 gl-1-2- 22 gL-1-4- 42 gL-1

At 300 K 36 g of glucose present in a litre of its solution has an osmotic pressure of 4.98 bar.

Class 12 Chemistry Chapter 2 NCERT Solutions PDF Download

2) 35.0 II. (1) 22.4 lit. (3) 448 lit. (4) 44800 ml Toleo At S.T.P., the density of nitrogen monoxide is - (2) 30 GL-1 (1)3.0 GL-1 (3) 1.34 gl-1 (4) 2.68 gL-1

At 300 K, 36 g of glucose present per litre in its solution has an osm

At 300K,36g of glucose present per litre in its solution had an osmoti

2.22At300 K,36 g of glucose present in a litre of its solution has an osm..
NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions

At 300K,36g of glucose present per litre in its solution had an osmoti
⏩SOLVED:At 300 K, 36 g of glucose present per litre in its…

At S.T.P., the density of nitrogen monoxide is - (1) 3.0 GL-1 (2) 30 GL-1 (3) 1.34 L-1 (4)2.68 gL-1
- DORINA Claire - Bubbleroom

- Braided Leather Waist Cincher / Corset Belt customizable With Your Choice of Leather and Metal Color - Canada

- Peacock Blue Pencil Women Shapewear Skirt, Ladies Cotton with Drawstring Saree

- BRATZ GENIE MAGIC: Sasha

- Peyakidsaa Women Jeans Streight Wide-Leg Mid Waist Loose Baggy





)