Example of a primary drying design space graph showing sublimation
By A Mystery Man Writer

Recommended Best Practices for Lyophilization Validation—2021 Part I: Process Design and Modeling
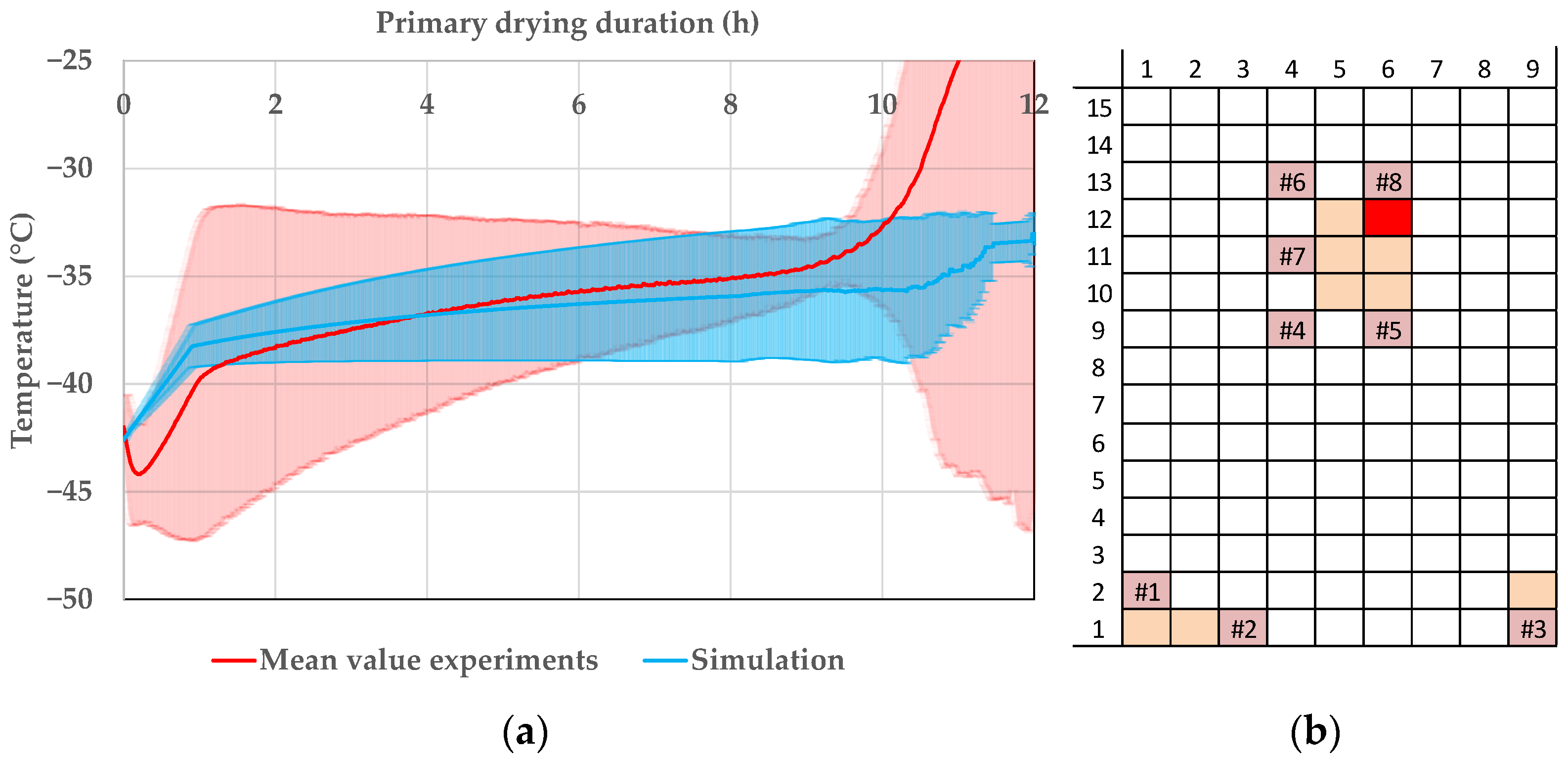
Pharmaceutics, Free Full-Text

PDF) Advanced approach to build the design space for the primary drying of a pharmaceutical freeze‐drying process

Freeze-drying parameters for uncontrolled and controlled

Ehab MOUSSA, Senior Scientist, PhD, AbbVie, Illinois
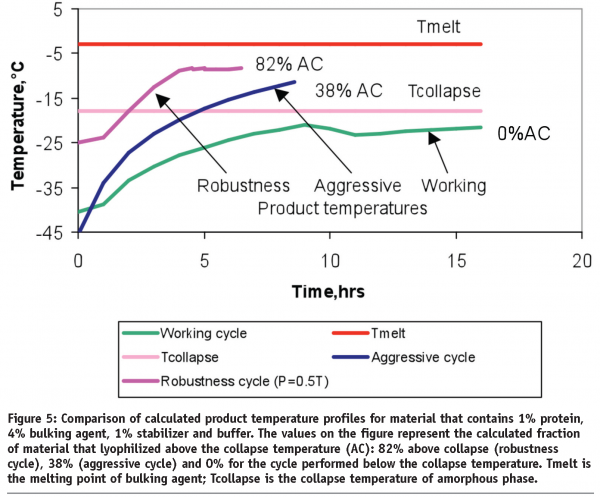
Lyophilization: cycle robustness and process tolerances, transfer and scale up - European Pharmaceutical Review

Gregory SACHA, Senior Research Scientist

PDF) Recommended Best Practices for Lyophilization Validation—2021

Full article: Model development for the design of control strategies of the primary drying of lyophilization in vials

Gregory SACHA, Senior Research Scientist

Gregory SACHA, Senior Research Scientist

Choked flow and importance of Mach I in freeze-drying process

Determination for dry layer resistance of sucrose under various primary drying conditions using a novel simulation program for designing pharmaceutical lyophilization cycle - ScienceDirect

PDF) Advanced approach to build the design space for the primary drying of a pharmaceutical freeze‐drying process

PDF) Recommended Best Practices for Lyophilization Validation—2021
- Dye-sublimation printing: benefits of this technology

- Dye Sublimated Graphics for The Hockey Shop - Langley, BC - Cowan

- A Word on Sublimation and Dye Migration

- T Shirts : Dye Sublimation Specialty Items : Products : Laurel Print & Graphics

- Men's Kona Triathlon Race Suit with Sublimated Graphics – Kona Tri Apparel





