Calculate the number of molecules of CO_2 present in 4.4 g of it.

By A Mystery Man Writer
Click here:point_up_2:to get an answer to your question :writing_hand:calculate the number of molecules of co2 present in 44 g of it
Click here👆to get an answer to your question ✍️ Calculate the number of molecules of CO-2 present in 4-4 g of it

CALCULATE THE MASS OF NITROGEN WHICH CONTAINS SAME NUMBER OF MOLECULES AS ARE PRESENT IN 4.4GM OF CO2?

Consider the reaction: 4 HCl(g) + O2( g)¡2 H2O(g) + 2 Cl2( g) Eac

Calculate mass of Nitrogen (N2) which contains same number of molecules as are present in 4.4g of carbon

SOLVED: Solve the following numericals: (10M) 1. Calculate the number of particles in each of the following: a) 0.124 g of Mg atoms b) 18 g of O2 molecules 2. Convert into

Calculate mass of nitrogen n2 which contains same no Of molecules as are present in 4 4 g of - Science - Atoms and Molecules - 12397645
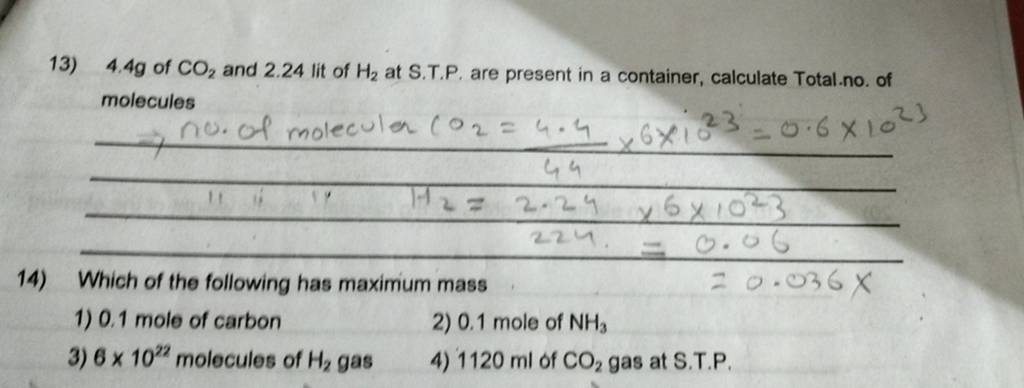
4.4 g of CO2 and 2.24 lit of H2 at S.T.P. are present in a container, ..

d) A flask contain 4.4g of CO2 gas. Calculate- (i) How many moles of CO2 gas does it contain? (ii) How many molecules of CO2 gas are present in the sample? (atomic
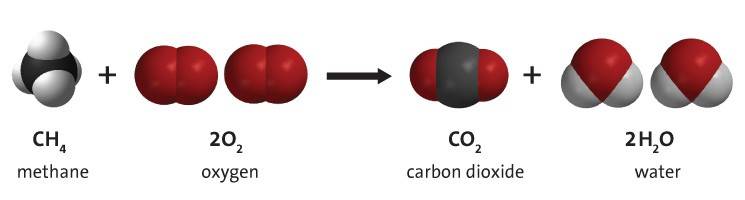
Lesson 6.1: What is a Chemical Reaction? - American Chemical Society

CH150: Chapter 4 - Covalent Bonds and Molecular Compounds - Chemistry
- 2023 Toyota Tundra with 22x12 -44 G-FX Tm6 and 305/45R22 General Grabber Uhp and Leveling Kit

- NOS TAMANHOS 40(P) E 42( M) BOJO COM BOLHA . 44 (G) E 46 (GG) BOJO

- Bala Mastigável Macia Sabor Morango My Chew - 44 gramas - Hachi8

- Fralda Descartável Turma da Mônica Baby G 44 Unidades - Drogaria Sao Paulo

- Botana Sabritas Paketaxo Mezcladito chile, sal, limón y especias

- 8 Lace Top Outfit Ideas - the gray details

- Buy White Recycled Lace Full Cup Comfort Bra - 40C | Bras | Argos
- Adhesive Bra Women 𝙸nvisible Push Up Strapless Breast Lift Breast Lifting Silicone Bra (Black, B)

- Fashion Women Postpartum Shapewear Wirefree Nursing Bra Cotton Breastfeeding Underwear Vest Maternity Clothes Pregnancy Tank Top @ Best Price Online
/product/97/501116/1.jpg?0123)
- Spanx Fabulous Footless Tights High Waisted Nude Womens Plus Size F Shapewear

