SOLVED: For a gas at a given temperature, the compression factor is described by the empirical equation: z = 1 - 8.50 × 10^(-3)P/P° + 3.50 × 10^(-5)(P/P°)^2 where P° = 1

By A Mystery Man Writer
VIDEO ANSWER: Hello students: let's look at the question: l n, that integrate integration and 0 z minus 1 bracket, close d p by p here. Minus 1 is equal to minus 8.50 into 10 to the power minus 3 p by p, not plus 3.50 into 10. To the power minus 9. P
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

Schaum's Outlines - 3,000 Solved Problems in Chemistry, PDF, Chemical Bond

PDF) Compact Reversed-Field Pinch Reactors (CRFPR): preliminary engineering considerations
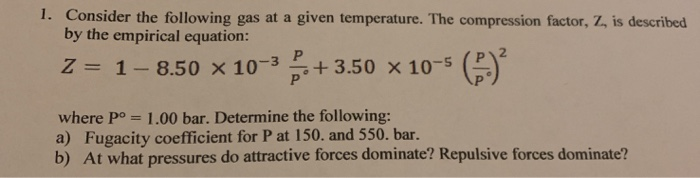
Solved 1. Consider the following gas at a given temperature.

Mean value first principle engine model for predicting dynamic behaviour of two-stroke marine diesel engine in various ship propulsion operations - ScienceDirect

Solved Real gas effects can be expressed as departures from

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!

Thermodynamics: Ideal Gas EOS and Compressibility Factor

Introduction to Fluid Mechanics and Fluid Machines [2 ed.] 0070667624, 9780070667624
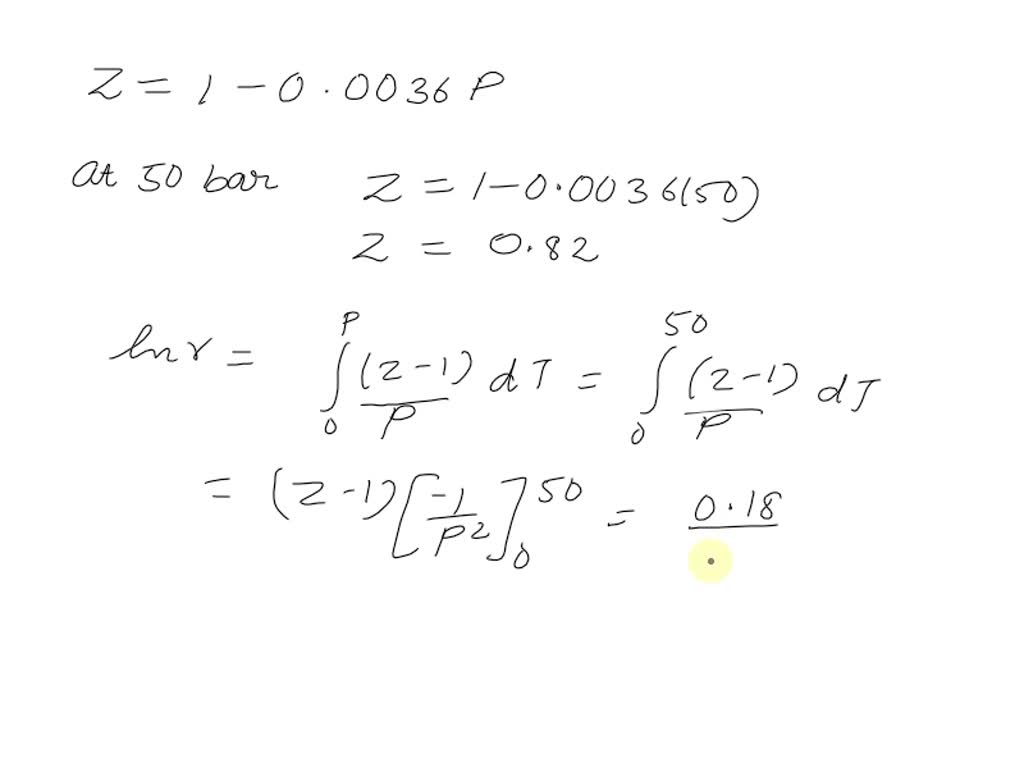
SOLVED: PROBLEM 3: The compressibility factor (Z) for nitrogen was measured at -100 °C from 10 to 50 bar pressure. The results are shown below: Pressure (bar) Compressibility factor (Z) 0 14
SOLVED: Isopentane (CH3-CH(CH-CH-CH3) is a hydrocarbon compound that is common within gasoline. Isopentane has a critical temperature (Tc) = 460.4 K, critical pressure (Pc) = 33.81 bar, and acentric factor (ω) =
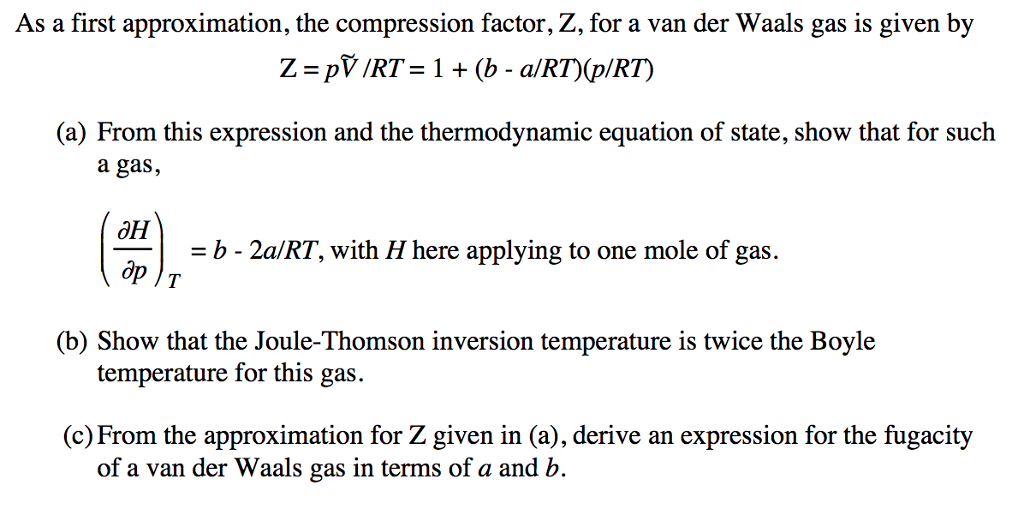
- Solved As a first approximation, the compression factor, Z
- At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry

- Solved 1. Consider the following gas at a given temperature.
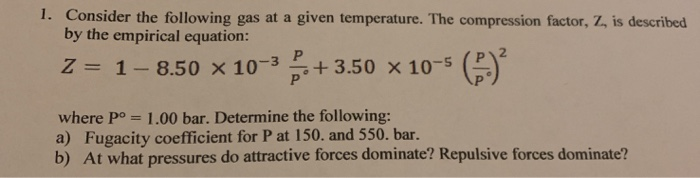
- 53 pts!! The function f(x)= 7^x+1 is transformed to function g through a horizontal compression by a factor

- The Compression Factor, Z, and Real Gases - What you NEED to Know

- Jingle Bells To Wedding Bells - Frosted Cups - Yippee Daisy

- Fabletics, Pants & Jumpsuits, Fabletics Navy Blue 247 Kick Flare Pant Size Medium

- Sexywg Butt Lifter Shaper Panties Women Hip Shapewear Sexy Shapewear Push Up Panties Body Shaper Hip Enhancer Panties

- BTMWAY Lift Chairs for Elderly, Electric Power Lift Recliner with Heat Therapy and Massage Function, Heavy Duty Recliner Sofa with Cup Holders, USB

- Looking for the perfect blue that won't make me think of my uniform skirt 🤣 - should I just keep both? : r/lululemon
