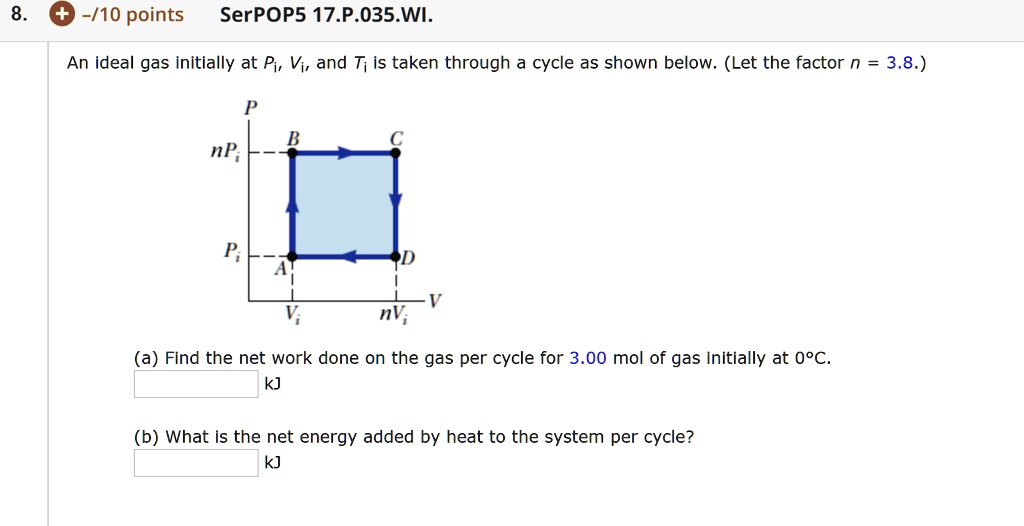
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through
By A Mystery Man Writer
VIDEO ANSWER: Hello. Here we are given a PV diagram. So it's in shape of a square. Right? And the process and volume R P I If you have N B I. Here we have A V I. And here we have N. B I. Right? And it's given that N is equal to 3.6. So an ideal
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

1 mole of an ideal gas undergoes reversible isothermal expansion from an initial volume V_{1} to a final volume 10V_{1} and does 10 KJ of work. The initial pressure was 1times 10^{7}PaCalculate V_{1}
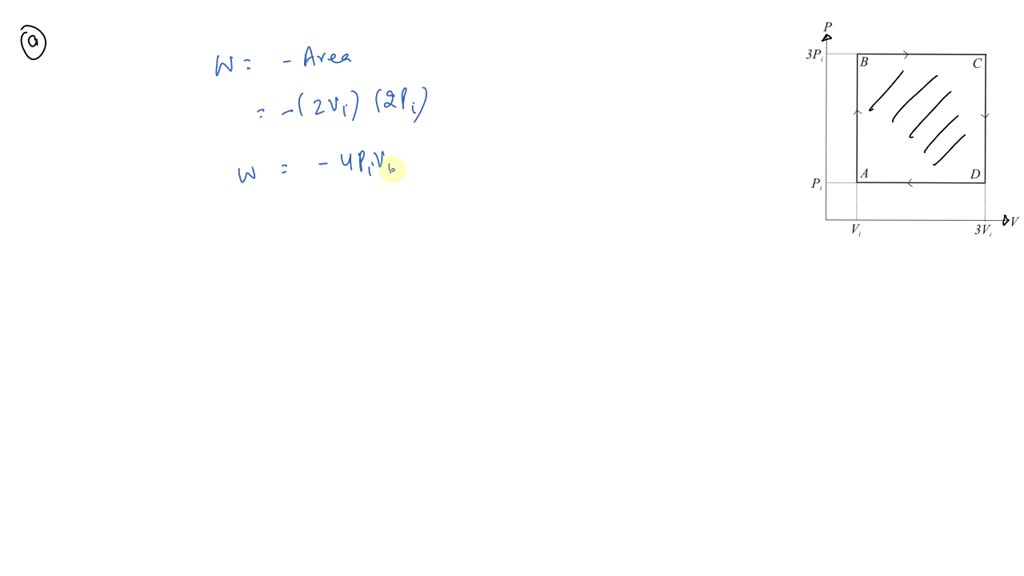
⏩SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…

Solved 8P An ideal monatomic gas initially at P;= 2 atm, Vi=

Molar Mass & Ideal Gas Law, Overview, Formula & Examples - Lesson

In the given figure an ideal gas changes its state from `A` to state `C` by two paths `ABC` and
Let's Derive the Ideal Gas Law from Scratch!
Energy Equation & Bernoulli's Equation – Introduction to Aerospace Flight Vehicles

A 1.00 mol sample of monoatomic ideal gas is take through the cycle shown. At point A, the pressure, volume and temperature are P_i, V_i and T_i respectively. In terms of R

An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most. - Physics

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown in the figure below where n = 2. (Use any variable or symbol stated above
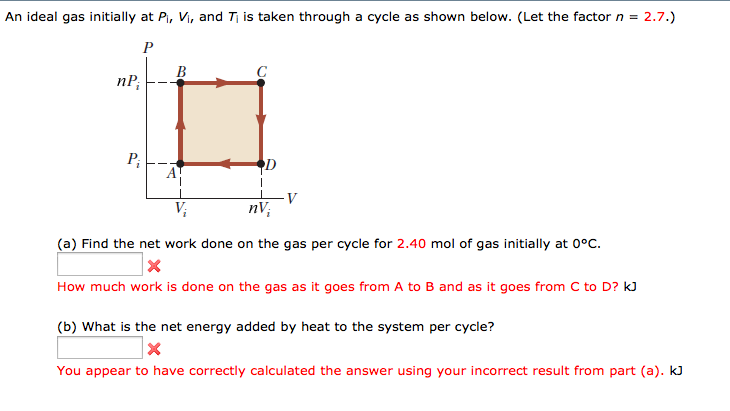
An ideal gas initially at Pi, Vi, and Ti is taken
- A fun way to celebrate Pi-Day! Each student designs a Pi Day t-shirt using the pi symbol and the word pi! Some ideas include: V…

- 10.3inch e-Paper e-Ink Display HAT, 2-16 Grey Scales,for Raspberry Pi,Ideal for e-reader, eink monitor, industrial instruments.. - AliExpress

- PI Ideal - S / Natural
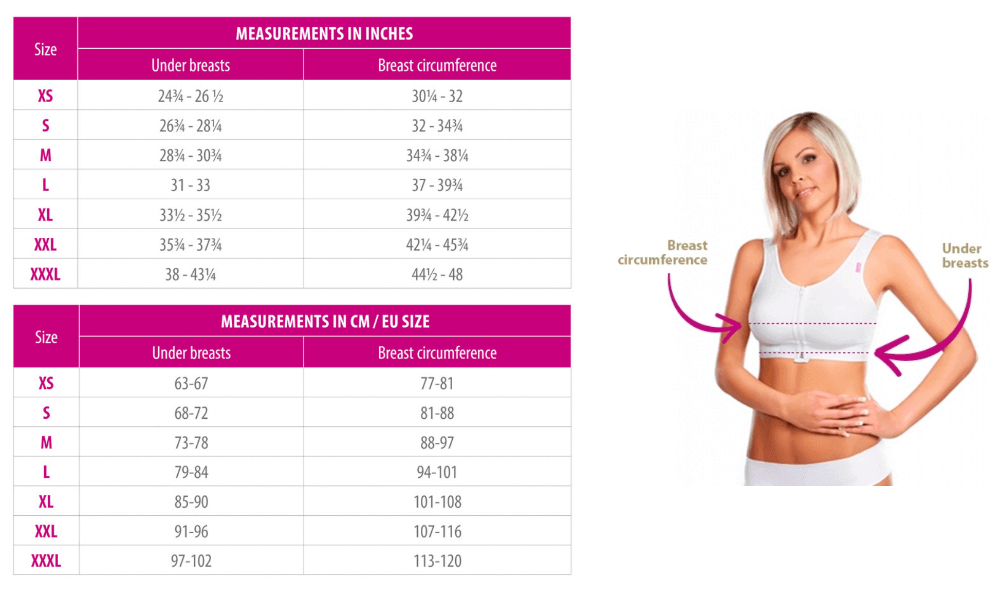
- Biustonosz Kompresujący Lipoelastic PI Ideal Variant - Ceny i opinie

- BitScope Micro Raspberry Pi Oscilloscope!
- Patchwork Quilts and Bedspreads - Bed Bath & Beyond

- 1 pieza de mallas de compresión transpirables para mujer, mallas

- Dickies EDS Essentials Women's Drawstring Cargo Scrub Pant DK010 - Netuniform
- Suanret Plus Size Women Posture Corrector Bra Yoga Bras Underwear Fitness Bras

- Oversized Carved Wood Tray - Hearth & Hand™ With Magnolia : Target

