Solved An ideal gas initially at Pi, Vi, and Ti is taken
By A Mystery Man Writer

A 1.00 mol sample of monoatomic ideal gas is take through the cycle shown. At point A, the pressure, volume and temperature are P_i, V_i and T_i respectively. In terms of R

In the given figure an ideal gas changes its state from `A` to state `C` by two paths `ABC` and
Solved An ideal gas initially at Pi,Vi, and Ti is taken

What is the Maxwell-Boltzmann distribution? (article)

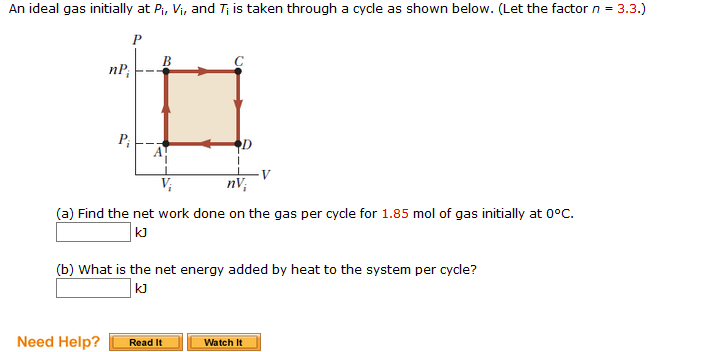
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown in the figure (Pressure in Pa and Volume in m^3) What is the net energy
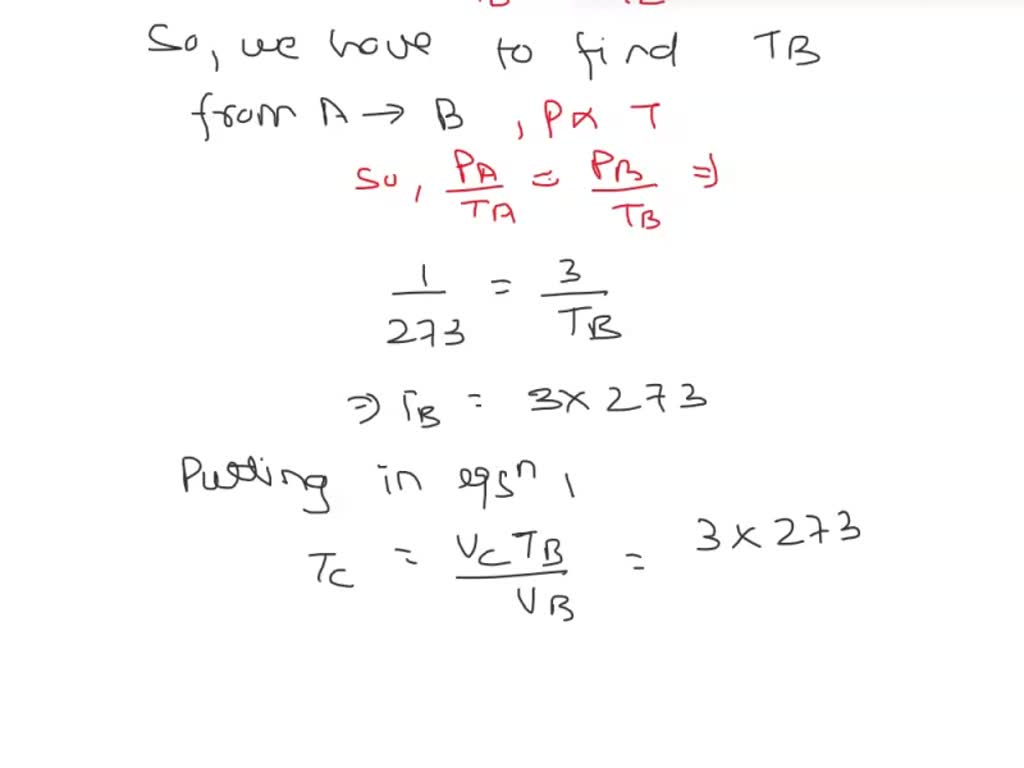
SOLVED: 3P; 3Vi A mole of ideal gas initially at Pi-l Pa, Vi-S m³, and Ti= 0°C is taken through a cycle as shown in the above Figure. a) Find the temperature

An ideal gas is taken around the cycle `ABCA` shown in `P - V` diagram. The net work done by the

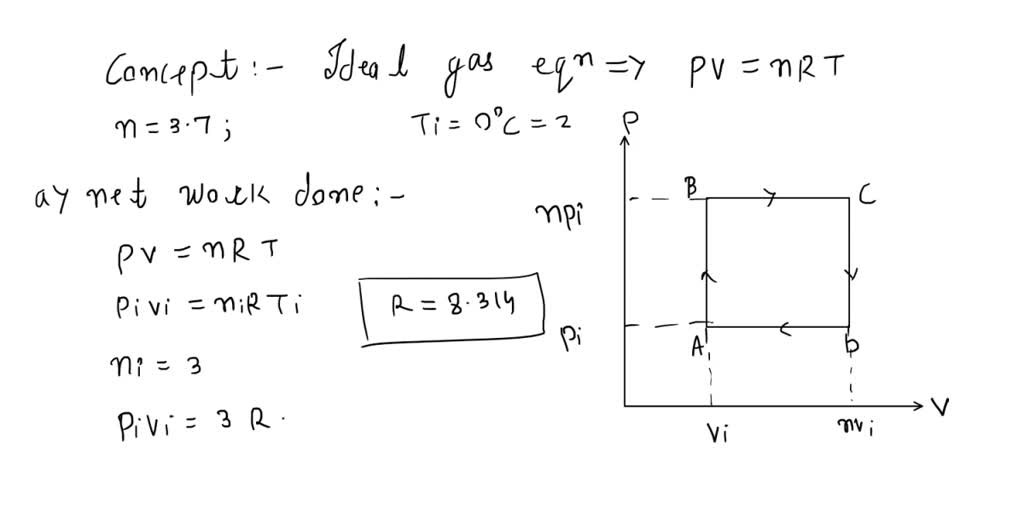
SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through a cycle as shown below. (Let the factor n = 3.1.) (a) Find the net work done on the

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of
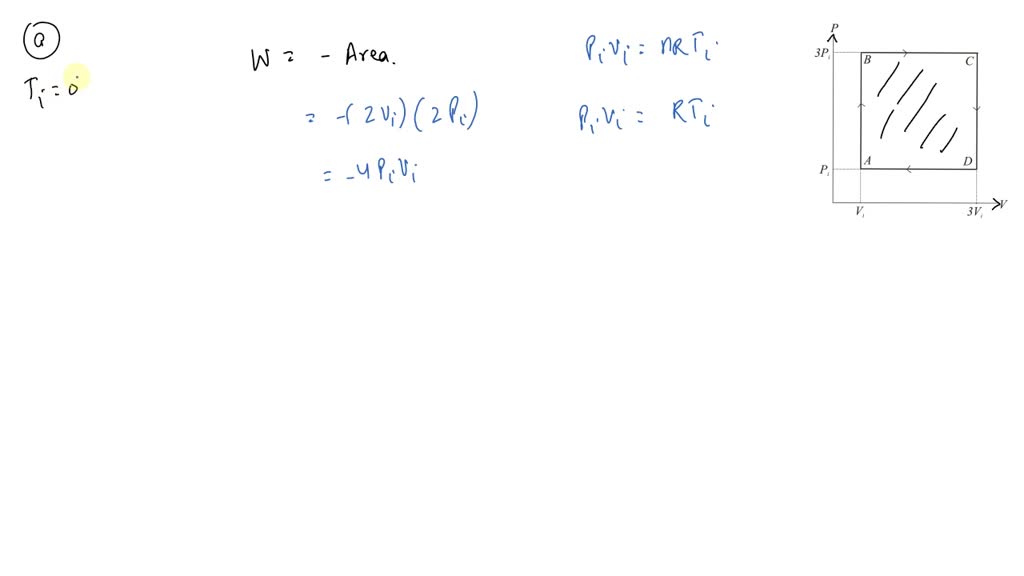
⏩SOLVED:An ideal gas initially at Pi, Vi and Ti is taken through a…
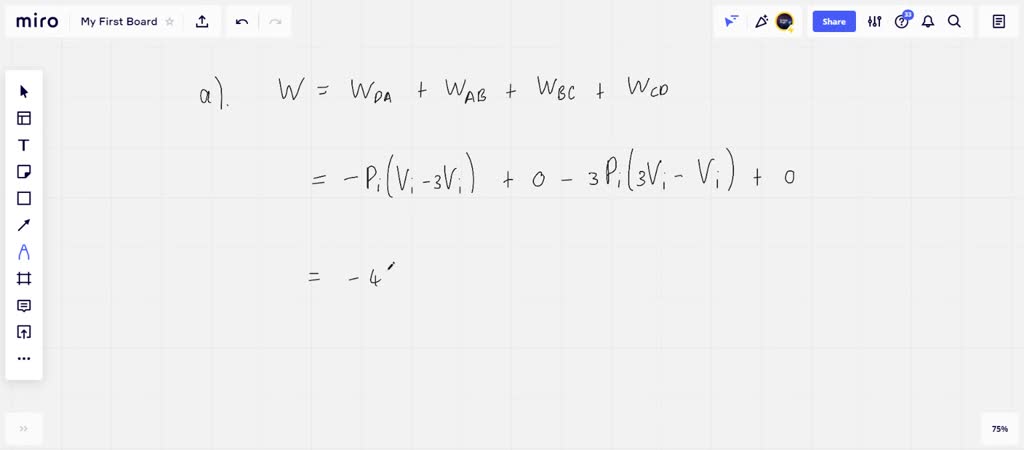
SOLVED:An ideal gas initially at Pi, Vi, and Ti is taken through a…
- Lipoelastic PI Plus Compression Bra – Sieden

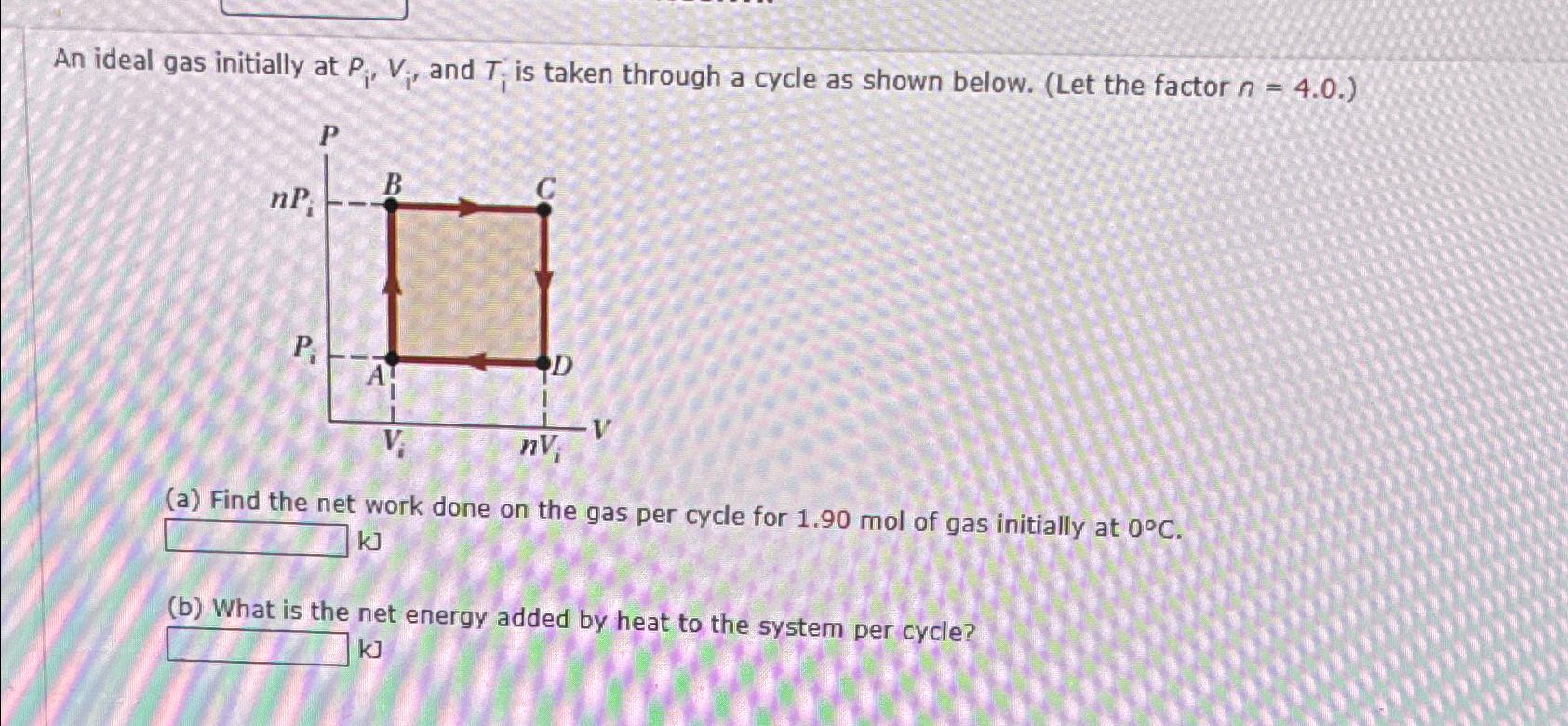
- SOLVED: ideal gas initially at Pi, Vi, and Ti is taken through cycle as shown below: (Let the factor n 3.7.) nf Find the net work done on the gas per cycle
- Pi day idea for a poster or postcard Royalty Free Vector

- The 16 relay module and the Raspberry Pi: not an ideal marriage – Arduino, ESP8266, ESP32 & Raspberry Pi stuff

- Lithium Ion Polymer Battery Ideal For Feathers - 3.7V 400mAh

- Back Support Neoprene Magnetic Shoulder Belt Lumbar Support Breathable Pain Medical Posture Corrector (Pink, 2XL)

- 36c Bra Pack Sport Bras Padded Strappy Cropped Bras for Yoga

- Buy Wholesale China 2021 Yoga Set Plus Size Workout Clothes Eco 2 Piece Scrunch Butt Fitness Big Size Yoga Wear & Big Size Yoga Wear at USD 10.1

- Keratin Hair Mask Purple Hair Mask Removes Yellow Tones Reduces

- Women's Lace Bodysuit Shapewear With Built-In Bra Body Shaper Butt
