For a given gas, a graph is shown between compressibility factor

By A Mystery Man Writer
By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.

Left panel: Compressibility factor Z of the gas consisting from the

Real-gas z-factor, as attributed to Standing and Katz, 9 plotted

i want to know the definition of compressibility factor ,how is it

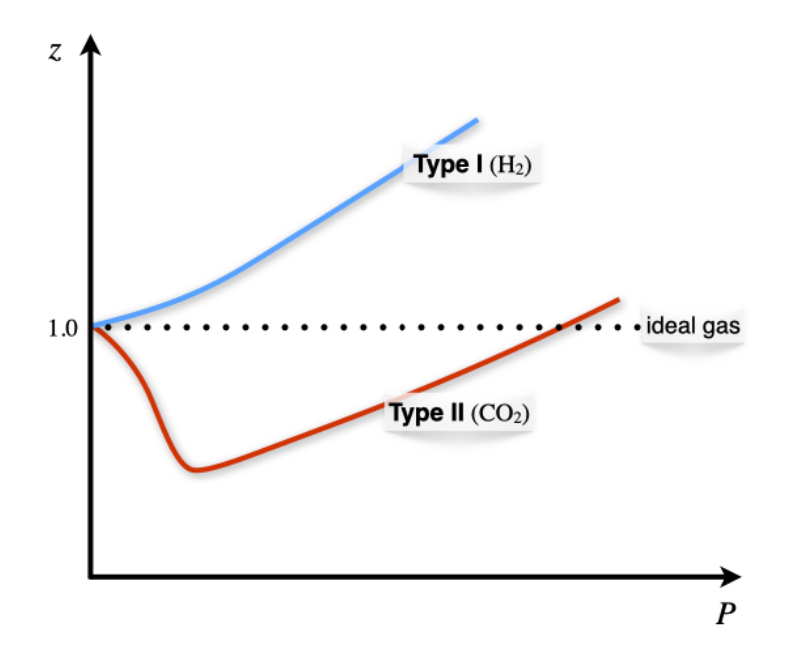
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

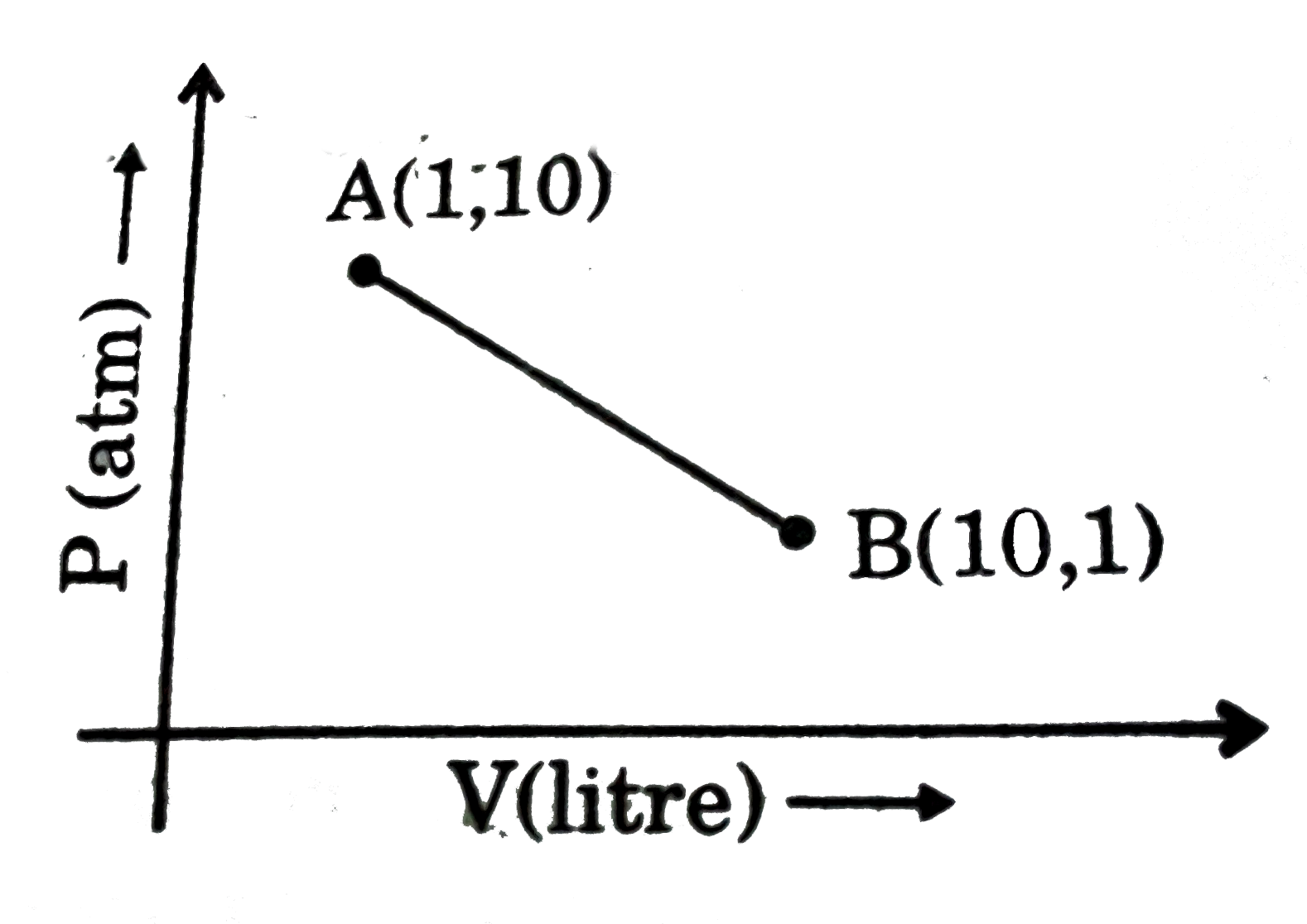
For a reaction,the plots of [A] and [P] with time at temperatures T1and T2are given below.If T2andgt;T1, the correct statement(s) is (are)(AssumeHandtheta;and Sandtheta;are independent of temperature and ratio of lnK at T1to
11.3: Critical Phenomena - Chemistry LibreTexts
Punjabi] The graph of compressibility factor (Z) vs. P for one mole o

Non-Ideal Gas Behavior Chemistry: Atoms First

Compressibility factor (z): real gases deviate from ideal behav-Turito
- Compressibility Factor Z
- Explain how the compression factor varies with pressure and

- Compressibility Factor Z for sub-critical pressures for Lee

- plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

- Gas Compressibility Factor and Control Valve Sizing





