Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an

By A Mystery Man Writer

Energies, Free Full-Text
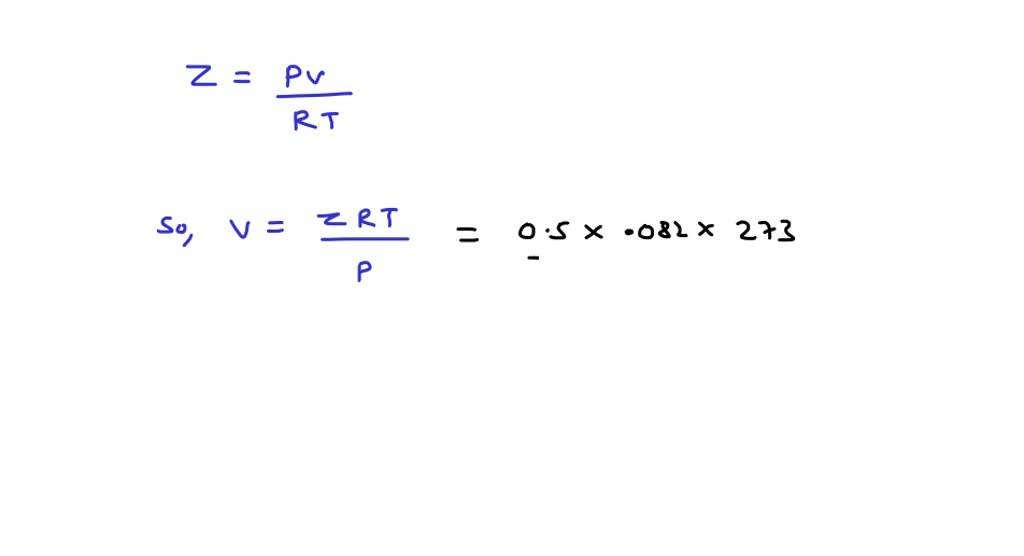
⏩SOLVED:Compressibility factor for 1 mol of a van der Waals gas at…
Gas Laws – First Year General Chemistry
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

SOLVED: 4.17 The non-ideality of a gas may be expressed as a compressibility factor, z: PVm RT a. Find the value of z for the ideal gas. b. Given the van der

EGR 334 Thermodynamics Chapter 3: Section ppt video online download

Solved We begin by showing that the compressibility factor

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Ideal Gas vs. Real Gas - Chemistry Review (Video)

Deviation from ideal gas behaviour

SOLVED: 4.17 The non-ideality of a gas may be expressed as a compressibility factor, z: PVm RT a. Find the value of z for the ideal gas. b. Given the van der

Problem Set 2 Solutions
Gas Laws – First Year General Chemistry
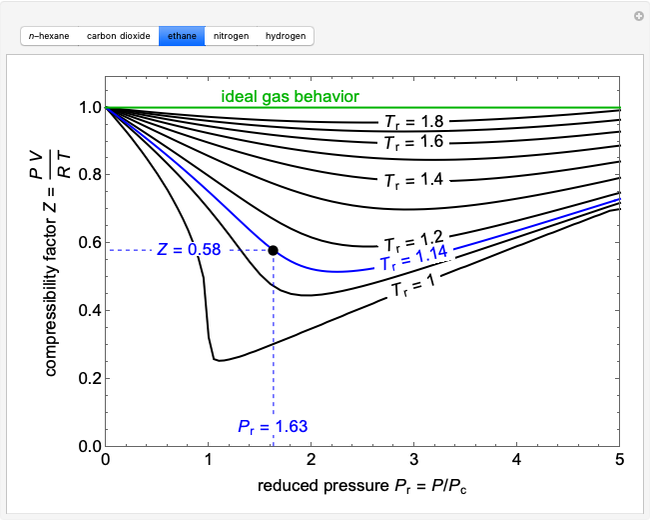
- Compressibility Factor Charts - Wolfram Demonstrations Project
- What is the compressibility factor (Z) for 0.02 mole of a van der

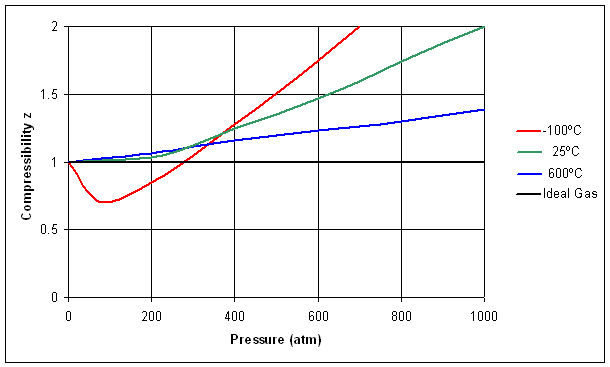
- The given graph represent the variations of Z Compressibility

- gas laws - How to find the temperature relationship between the

- In the above figure, near the point B, compressibility factor Z is about..
- Cast Iron Heavy Vehicle Bevel Helical Gearboxes, For Automobile Industry at Rs 5905 in Hyderabad

- RELAX! Propolis & Honey Soothing Moisturizer Face Cream for Rosacea Sensitive Skin – Hey Honey Beauty

- SPANX Perfect Length Dolman 3/4 Sleeve Top, Plus Size 3XL

- DOULAFASS Women Butt Lift Leggings Seamless Gym Leggings Scrunch

- Baocc Yoga Pants Women Soft Solid Stretch Cheerleader Running
