Quantum Numbers for Atoms - Chemistry LibreTexts
By A Mystery Man Writer
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is …
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. Each electron in an atom has a unique set of quantum numbers; according to the Pauli Exclusion Principle, no two electrons can share the same combination of four quantum numbers.

Quantum Numbers for Atoms - Deepstash
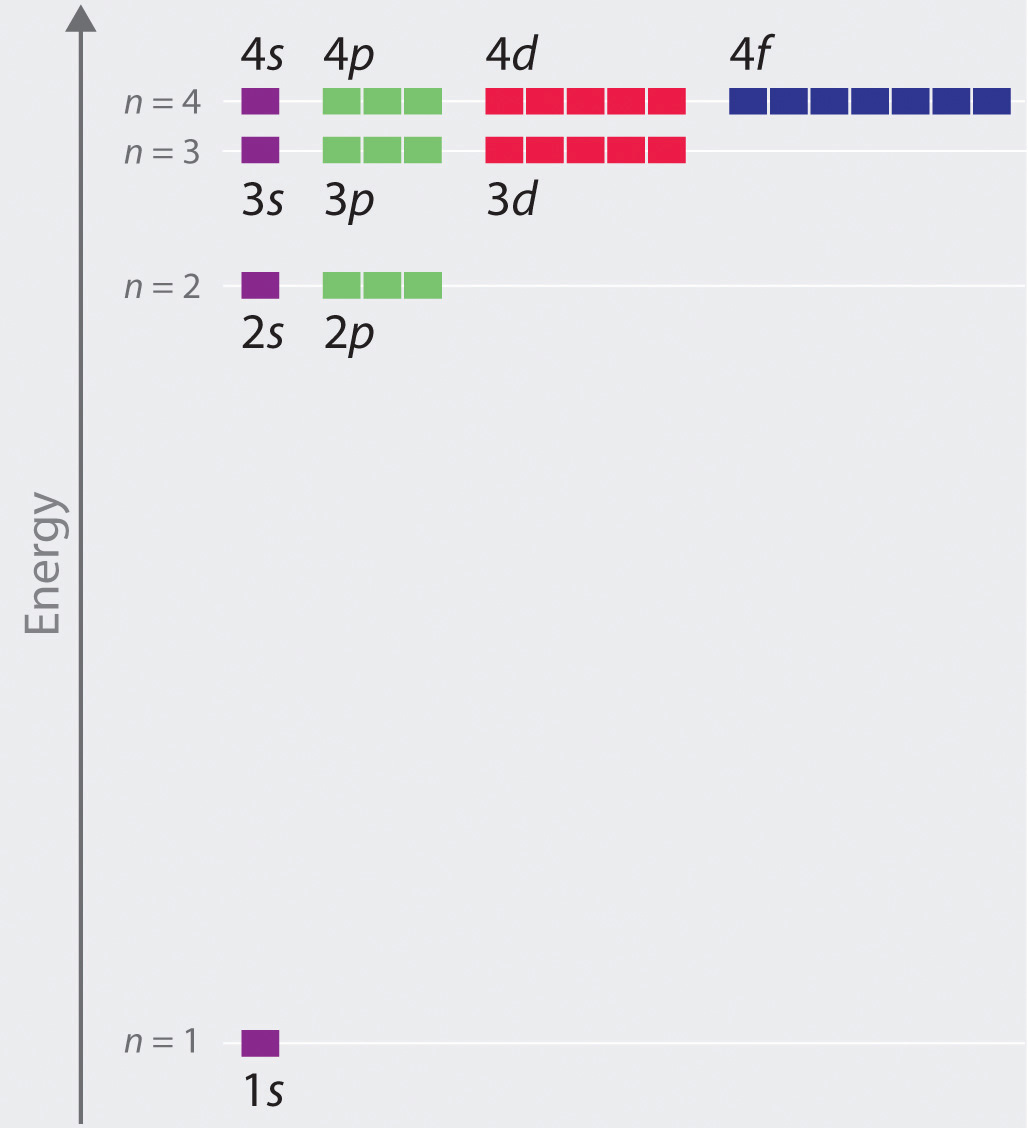
11.10: The Schrödinger Wave Equation for the Hydrogen Atom

Las shs gen.chem-melc_1_q2_week-1

1.4 - Introduction To Spectroscopy - Chemistry LibreTexts

Transition metal Definition, Properties, Elements, & Facts

Las shs gen.chem-melc_1_q2_week-1
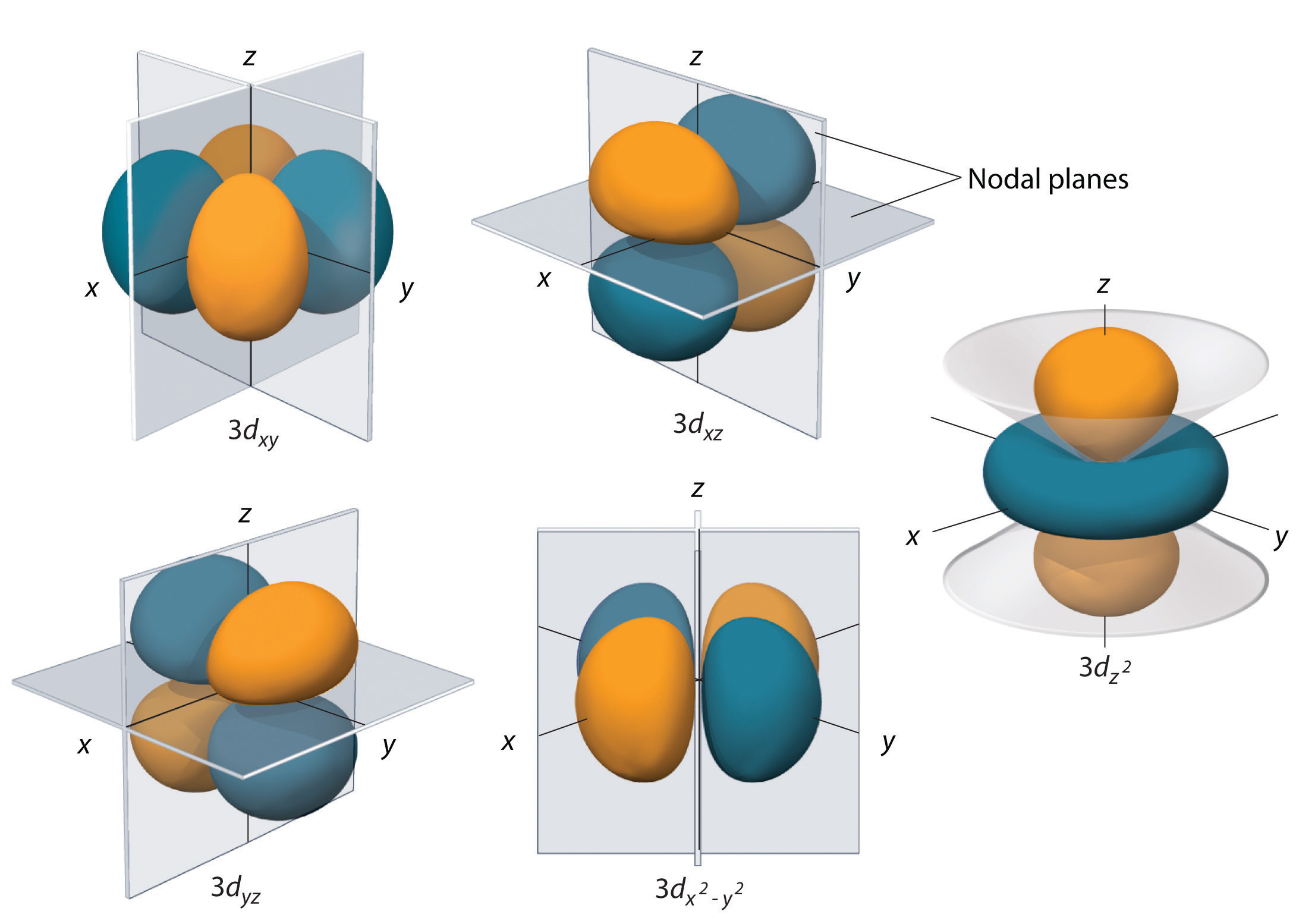
Question #fb349

The Quantum Numbers Understanding Atomic Structure
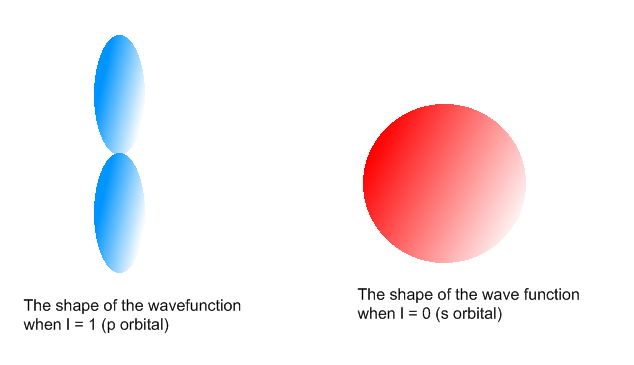
Quantum Numbers for Atoms - Chemistry LibreTexts
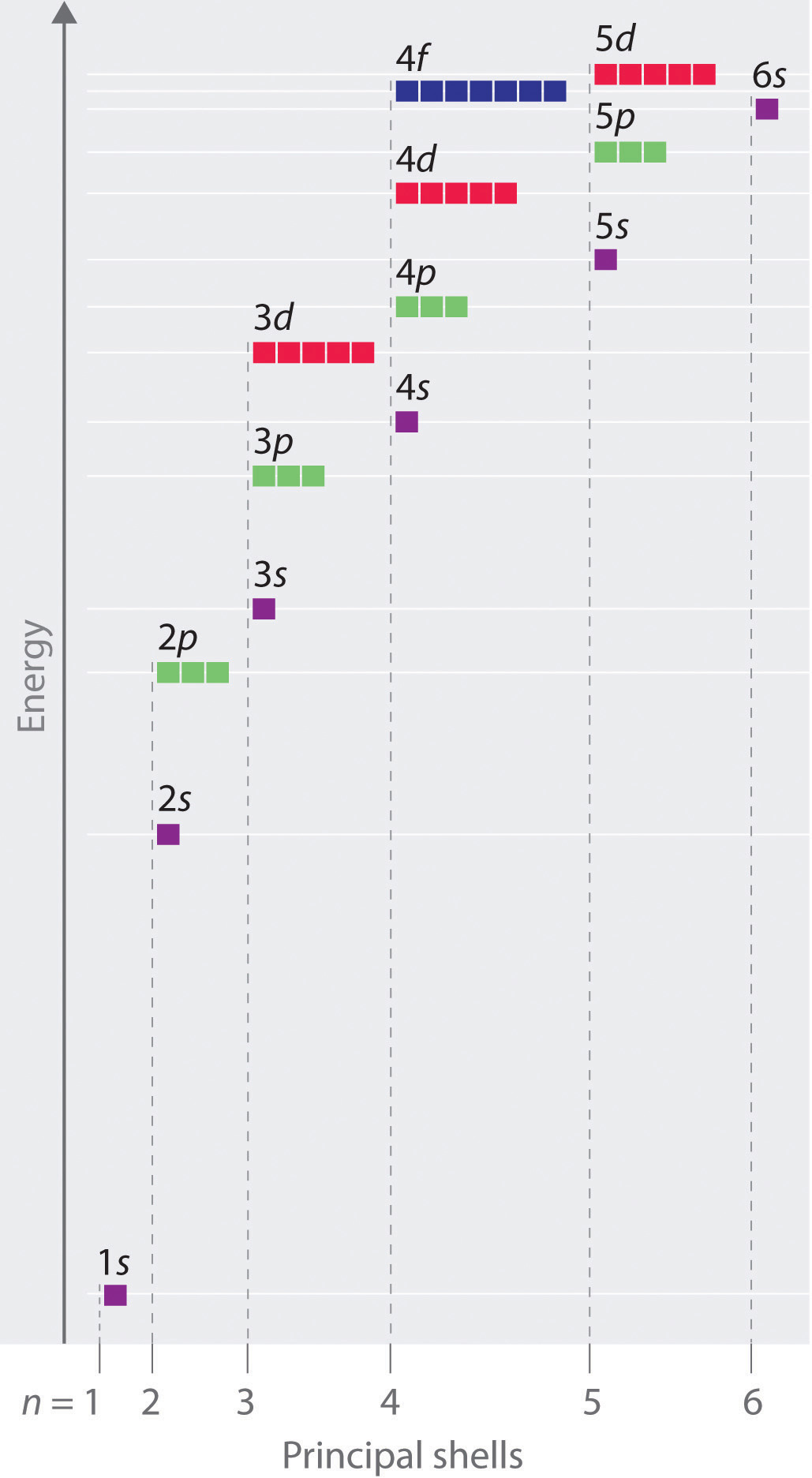
Chapter 2.5: Atomic Orbitals and Their Energies - Chemistry LibreTexts